SuperSep™ Phos-tag™ Precast Gels
SuperSep™ Phos-tag™ is a pre-cast gel copolymerized with Phos-tag™ acrylamide. It can be used immediately after opening the package, and enables isolation of phosphorylated proteins by the level of phosphorylation. It also has excellent storage stability owing to the use of a neutral gel buffer as with conventional SuperSep™, and results in sharp bands.
- Before using the product, it is important to perform conventional SDS-PAGE to ensure that there is no abnormal pattern of electrophoresis (e.g. confirm whether the applied amount is appropriate and that the target protein is not degraded). To obtain data for comparison, we recommend the use of SuperSep™ with the same concentration of acrylamide (refer to the Applications section).
Features
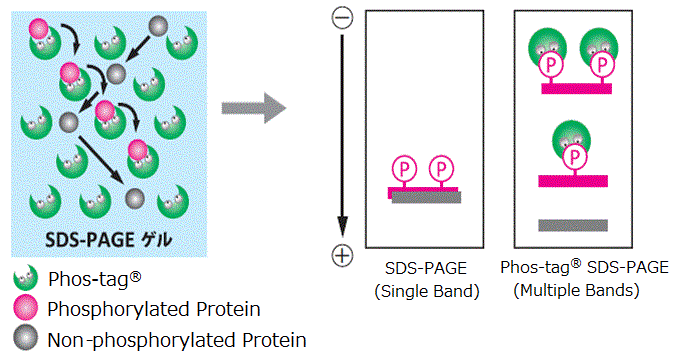
- Ready-to-use, saves time
- Able to isolate phosphorylated proteins by the level of phosphorylation
- Good separation with sharp bands
Applications
Albumin Dephosphorylation
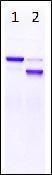
[Electrophoresis Buffer] Tris-Glycine SDS Buffer
[Electrophoresis Samples]
Lane1: Untreated Albumin
Lane2: Dephosphorylated Albumin
[Electrophoresis cConditions] 20 mA, 70 mins
[Staining] Quick CBB staining
[Destaining] Deionized wWater
Albumin (Cat. No. 010-17071) was dephosphorylated using alkaline phosphatase (NIPPON GENE CO., LTD., Cat. No. 319-02661). Band shift confirmed dephosphorylation of albumin.
Time-dependent Dephosphorylation of β-casein
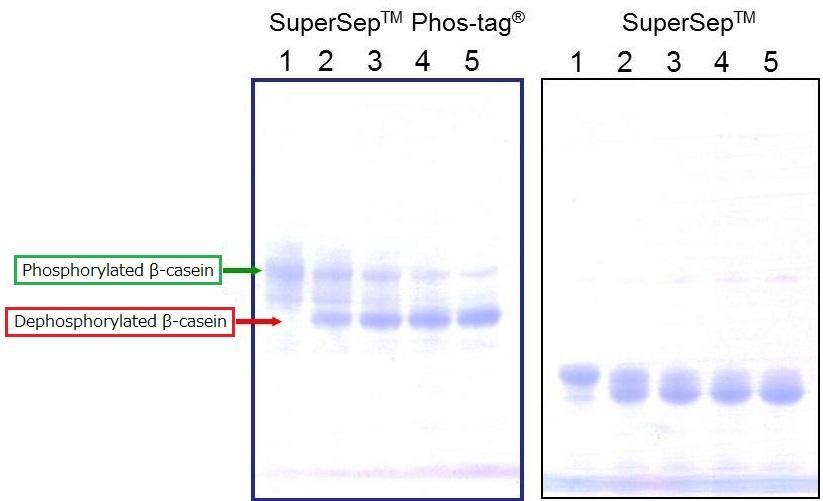
[Electrophoresis Buffer] Tris-Glycine SDS buffer
[Electrophoresis Samples]
Lane1: β-casein (AP-treatment: 0 min)
Lane2: β-casein (AP-treatment: 15 min)
Lane3: β-casein (AP-treatment: 30 min)
Lane4: β-casein (AP-treatment: 45 min)
Lane5: β-casein (AP-treatment: 60 min)
[Electrophoresis Conditions] 35mA, 60min
[Staining] Quick CBB staining
[Destaining] Deionized Water
Dephosphorylation of β-casein was performed over different time periods. The results confirmed separation of phosphorylated and dephosphorylated β-casein.
In addition, the results demonstrated the level of dephosphorylation across different time points.
Time-dependent Change in the level of phosphorylation
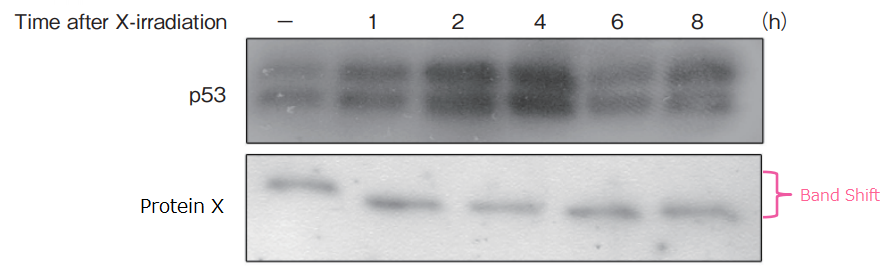
Procedure
X-ray irradiation (5 Gy) was performed for human lung cancer cell line Lu99, and cells were collected across different time points. Cell extracts were prepared, and SDS-PAGE was performed in 13 wells using 50 μmol/L of 10% SuperSep™ Phos-tag™
Gentle agitation of the gel was performed in a transfer buffer containing 10 mmol/L EDTA, and proteins were transferred to PVDF membrane. The membrane was blocked with 2% Milk/TBS-T, and was reacted with primary antibodies (upper image: p53, lower image: cell-cycle proteins). Detection was performed by chemoluminecence.
Results
Protein accumulation for p53 was at the highest level 4 hours after X-ray irradiation. The level of protein X phosphorylation changed over time after X-ray irradiation.
Electrophoresis of Control Protein
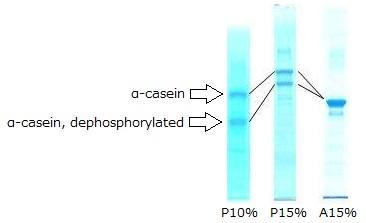
P10 %(left): SuperSep™Phos-tag™ (50 μmol/L), 10 %, 13Wells
P15 %(middle): SuperSep™Phos-tag™ (50 μmol/L), 15 %, 13Wells
A15 %(right): SuperSep™Ace, 15 %, 13Wells
Electrophoresis Conditions
30 mA/gel (constant), 60 mins
Sample
5 μg/lane of α-casein from bovine milk, dephosphorylated (Cat. No. 038-23221)
- The product contains α-casein that hasve not been dephosphorylated. While conventional SDS-PAGE will only show a single main band, Phos-tag™ SDS-PAGE will show two main bands for α-casein and dephosphorylated α-casein.
Running Buffer
Staining
Quick CBB Plus (Cat. No. 174-00553)
Correspondence table
For Bio-Rad Mini-PROTEAN® Tank
| 198-17981 | SuperSep Phos-tag (50μmol/l), 7.5%, 17well, 83×100×3.9 mm |
| 195-17991 | SuperSep Phos-tag (50μmol/l), 12.5%, 17well, 83×100×3.9 mm |
For Invitrogen® XCell SureLock Mini-Cell Tank
| 192-18001 | SuperSep Phos-tag (50μmol/l), 7.5%, 17well, 100×100×6.6 mm |
| 199-18011 | SuperSep Phos-tag (50μmol/l), 12.5%, 17well, 100×100×6.6 mm |
For FUJIFILM Wako Easy Separator™
| 192-17401 | SuperSep Phos-tag (50μmol/l), 6%, 13well |
| 199-17391 | SuperSep Phos-tag (50μmol/l), 6%, 17well |
| 193-16711 | SuperSep Phos-tag (50μmol/l), 10%, 13well |
| 190-16721 | SuperSep Phos-tag (50μmol/l), 10%, 17well |
| 195-17371 | SuperSep Phos-tag (50μmol/l), 7.5%, 13well |
| 192-17381 | SuperSep Phos-tag (50μmol/l), 7.5%, 17well |
| 195-16391 | SuperSep Phos-tag (50μmol/l), 12.5%, 13well |
| 193-16571 | SuperSep Phos-tag (50μmol/l), 12.5%, 17well |
| 193-16691 | SuperSep Phos-tag (50μmol/l), 15%, 13well |
| 196-16701 | SuperSep Phos-tag (50μmol/l), 15%, 17well |
Q&A
Q1. What electrophoresis tanks are the Phos-tagTM Precast Gels compatible with?
Phos-tag Precast Gels are available in three cassette sizes, [83 x 100 x 3.9 mm], [100 x 100 x 3.0 mm] and [100 x 100 x 6.6 mm].
- [83 x 100 x 3.9 mm] is suitable for Bio-Rad Mini-PROTEAN Tank.
- [100 x 100 x 3.0 mm] is suitable for Wako Easy Separator.
- [100 x 100 x 6.6 mm] is suitable for Invitrogen® XCell SureLock® Mini-Cell Tank.
Q2. What gel staining methods can we use?
CBB, negative, silver, and florescent staining methods can be used.
Q3. CBB-stained gel cannot be de-stained clearly.
A microwave can be used when de-staining to give you a clearer gel.
Procedure
Transfer the stained gel in 100 mL of deionized water in the container, add a few rolls of Kim-Wipes, and heat it in a microwave for a few minutes. Replace deionized water and repeat the same procedure 2-3 times. Be careful with handling as the container will be hot.
Q4. Can the product be used for Western blot?
Yes: However, zinc in the gel has to be removed by EDTA treatment to achieve optimal transfer efficiency to the membrane.
Procedure
Immerse the gel in a transfer buffer (25 mmol/L Tris, 192 mmol/L Glycine, 10% MeOH) containing 10 mmol/L EDTA, and agitate gently for 10 minutes. Repeat the procedure 2 times. Replace with a transfer buffer (25 mmol/L Tris, 192 mmol/L Glycine, 10% MeOH) that does not contain EDTA, agitate for 10 minutes, and transfer onto PVDF membrane or nitrocellulose membrane. If the transfer efficiency is poor, consider increasing the frequency of EDTA treatment or the concentration of EDTA.
Q5. Bands are distorted.
Distortion and tailing of bands can be observed with samples that contain EDTA, inorganic salts, and detergents. In such cases, it is effective to desalt samples by dialysis or precipitation with TCA. The presence of an empty lane can also cause band distortion. Apply a sample buffer (1x) in empty lanes to avoid distortion.
Q6. Phosphorylated and dephosphorylated target proteins are not separated.
Perform electrophoresis and confirm band shift with β-casein (positive control) and alkaline phosphatase-treated β-casein (negative control). If there is a band shift, it is likely that phosphorylated and dephosphorylated variants of your target protein are not separated with the Phos-tag™ and/or acrylamide concentrations of this product.
Q7. Can we use crude extracts of cells with this product?
Yes: However, the use of proteins with small Rf values may result in unclear bands.
Q8. What is the appropriate amount of a sample that should be applied to a well?
1-5 µg for purified proteins with CBB staining, and 10-30 µg for tissue or cell extracts (adjust the amount depending on the level of protein expression).
- These are only recommended amounts. SDS-PAGE or Western blot should be performed to determine the appropriate amount of a sample.
Q9. What molecular markers should we use?
There are no specific markers that we recommend as the product is not influenced by the molecular weight of a marker. Instead of a marker, E. coli -derived recombination proteins or dephosphorylated samples are recommended as negative controls.
Q10. Which ion complex is contained in the product?
The product contains zinc ion.
Q11. How can we determine whether the band shift is caused by phosphorylation?
Perform gel electrophoresis with 12.5% SuperSep™ Ace (Cat. No. 199-14971) with the same gel concentration to confirm that the target protein is not degraded.
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.
The prices are list prices in Japan.Please contact your local distributor for your retail price in your region.



