PYROSTAR™ ES-F series (LAL Gel-clot and Turbidimetric Reagent)
Endotoxin-specific LAL reagents (not activated by (1→3)-β-D-glucan), compatible with the BET (USP) compliance tests U.S Food and Drug Administration (FDA) licensed. Intended Use: Limulus amebocyte lysate (LAL) is intended for the detection of gram-negative bacterial endotoxins. PYROSTAR™ ES-F is intended for the qualitative detection of endotoxins by gel-clot or quantitative detection by kinetic turbidimetric methods.
- Endotoxin-specific lysate, avoids false positive results from glucans
- Available in multi-tests vials or single-test vials
- Can be used as either a gel-clot or Kinetic-Turbidimetric Assay (KTA) reagent
- KTA can be performed in tube reader
- Gel-Clot lysate sensitivities range from 0.015 to 0.25 EU/mL
- Available with matched control standard endotoxin (CSE)
- PYROSTAR™ ES-F reagents (Gel-clot sensitivity 0.015 EU/mL) are available with a KTA quantitative range of either 0.001 EU/mL to 10 EU/mL.
The KTA quantitative range is related to the Gel-Clot sensitivity. - Multi test is performed by mixing 0.1 mL of lysate reagent with 0.1 mL of sample, whereas in single test, a 0.2 mL sample directly dissolves lysate reagent.
| Single test: | The lysate reagent for one measurement is dispensed in advance in a test tube. |
|---|---|
| Multi test: | The lysate reagent which dissolve in the endotoxin test water, dispense the necessary amount in reaction tubes. |
PYROSTAR™ ES-F Single Test Vial
| Gel-clot Sensitivity(EU/mL) | KTA Quantitative Range(EU/mL) |
|---|---|
| 0.015 | 0.001 to 10 |
| 0.03 | 0.01 to 10 |
PYROSTAR™ ES-F Multi Test Vial with CSE
PYROSTAR™ ES-F Multi Test Vial without CSE (Bulk)
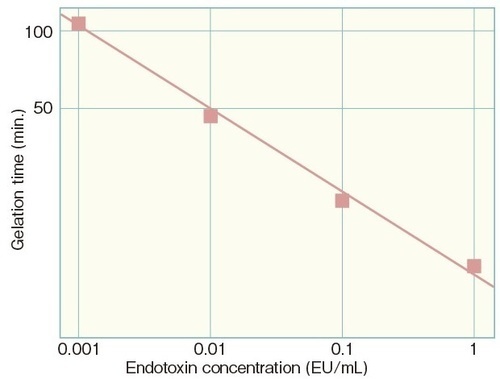
Standard curve information
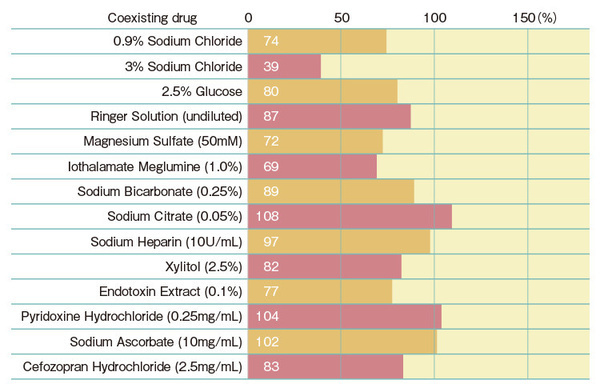
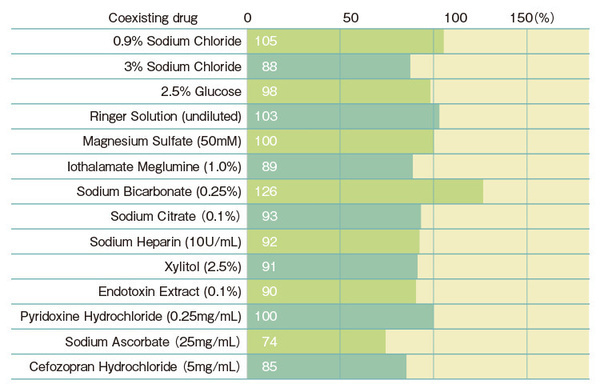
Recovery rate of spiked endotoxins with the Turbidimetric technique
*Multi test is performed by mixing 0.1 mL of lysate reagent with 0.1 mL of sample, whereas in single test, a 0.2 mL sample directly dissolves lysate reagent. Therefore, there will be a difference in sample concentration in the reaction solution. In general, single test tend to be more affected by samples.
Wako Blog: Talking of LAL
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.