MassivEV™ EV Purification Column PS / MassivEV™ Purification Buffer Set
Extracellular vesicles (EVs), such as exosomes, are small vesicles consisting of a lipid bilayer and are released by cells. EVs encapsulate nucleic acids, proteins, and lipids, serving as mediators of intercellular communication in living organisms. EVs are the subject of active research in various fields including immunology, cancer, neurology, and metabolism.
EVs have diverse applications, and are expected to be used as therapeutics, biomarkers, and drug delivery systems (DDS). In particular, EVs secreted by mesenchymal stem cells (MSCs) have attracted attention not only in the medical field but also in the cosmetics and food industries because of their anti-inflammatory, anti-fibrotic, and tissue repair effects.1)
The practical application of EVs requires technology capable of efficiently isolating and purifying high-purity EVs in large quantities. In collaboration with Prof. Hanayama of the Department of Immunology at the Graduate School of Medical Sciences, Kanazawa University, Fujifilm Wako has developed the MassivEV™ EV Purification Column PS, which utilizes our proprietary EV isolation and purification technology known as the PS affinity method.2) Used in conjunction with the specialized MassivEV™ Purification Buffer Set, this system enables convenient isolation and purification of EVs, including exosomes, from liter-scale cell culture supernatants.
-

MassivEV™ EV Purification Column PS
-
MassivEV™ Purification Buffer Set
Product Overview
The MassivEV™ EV Purification Column PS and MassivEV™ Purification Buffer Set have been specifically designed for the isolation and purification of extracellular vesicles (EVs), including exosomes, from large volumes of cell culture supernatants. This is achieved by utilizing the PS affinity method, which employs the Tim4 protein. Tim4 binds to phosphatidylserine (PS) on the surface of EV membranes in a calcium-dependent manner, making the purification of high-purity EVs a straightforward exercise.
Features
- High-purity EVs can be efficiently isolated and purified from large volumes of cell culture supernatants (from 10 mL to several liters)
- Eliminates the need for expensive equipment like tangential flow filtration (TFF) systems.* Only a peristaltic pump is required.
Table 1 Comparison of isolation and purification of EVs from 200 mL of MSC cell culture supernatant (evaluated by Fujifilm Wako)
| MassivEV™ | TFF + anion-exchange chromatography | TFF + size-exclusion chromatography | |
|---|---|---|---|
| EVs that can be purified | PS-positive EVs | Varies depending on fractions | Varies depending on fractions |
| Purity | High | Low | Low |
| Number of steps for purification | 1 step - PS affinity method |
2 steps - TFF system - Anion-exchange chromatography |
2 steps - TFF system - Size-exclusion chromatography |
| Yield of EV particles (reference value) | 1.7x1011 particles | 1.1×1011 particles | 0.7×1011 particles |
| Time required for purification | 8 hr | 10 hr | 10 hr |
Sample
Cell culture supernatant (e.g., MSC): From 10 mL to several liters
Processing Capacity
| 1 mL (Product No. 131-19491) | 5 mL (Product No. 137-19493) | |
|---|---|---|
| Sample volume*1 | 200 mL | 1 L |
| Dynamic binding capacity*2 | 5×1011 particles/mL resin | 2.5×1012 particles/5 mL resin |
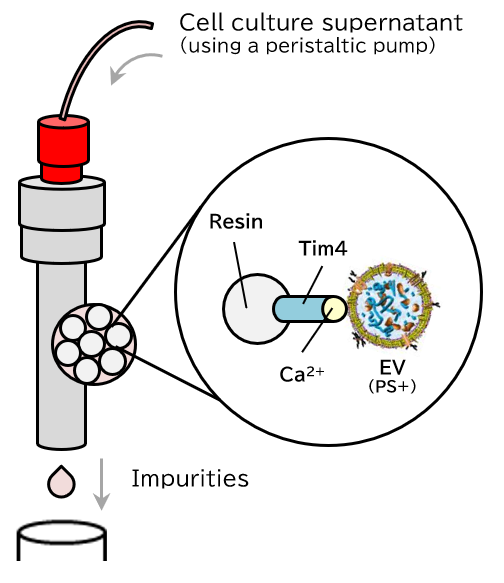
Principle
The PS affinity method, Fujifilm Wako’s proprietary EV isolation technique, utilizes the Tim4 protein, which specifically binds to phosphatidylserine (PS) present on the surface of EVs. The high specificity of the PS-Tim4 interaction, coupled with the gentle elution by a chelating agent, enables the isolation of high-purity EVs in their intact state.
-
Capture
-
Elution
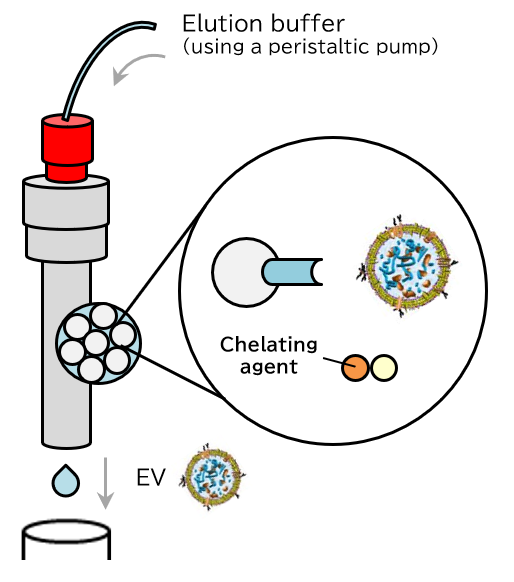
Figure 1 Principle of isolation and purification of EVs using the MassivEV™ EV Purification Column PS (PS affinity method)
Protocol
01
Culture the desired cells, facilitate cell proliferation and EV production, and collect the cell culture supernatant.
(>2 hrs)
02
Add EV Binding Enhancer to the cell culture supernatant.
03
Remove impurities by centrifugation or filtration.
04
Heat the cell culture supernatant so that it exceeds room temperature (25-28°C) and filter it through a filter.
(>7 hrs)
05
Bring the column to room temperature and pass the Washing Buffer through the column.
06
Pass the cell culture supernatant through the column, allowing the PS on EVs to react with the Tim4 protein on the resin.
07
Pass EV Binding Enhancer/Washing Buffer through the column to wash the resin.
08
Replace 50% of the EV Binding Enhancer/Washing Buffer in the column with EV Elution/EV-Stabilizer Buffer. Then, place an EV collection tube below the column and allow the EV Elution/EV-Stabilizer Buffer to flow through it to collect the EVs.
09
After flushing the column with Washing Buffer, circulate 20% ethanol/Storage Buffer through the column. Secure the top of the column with Parafilm and store in a refrigerator at 2-10°C.
(30 min to 1 hr)
10
Exchange the buffer using either gel filtration or size-exclusion filtration techniques.
For guidance on connecting the tubing, please refer the support document titled: Example of a purification system using the MassivEV EV Purification Column PS
[Protocol] MassivEV™ EV Purification Column PS
(Youtube 3:26)
Data
Performance Data
Comparison with conventional methods
Bone marrow-derived MSCs were cultured in the proliferation medium (MSCulture™/10% FBS) and then in the EV production medium (EV-Up™). The cell culture supernatant was collected and filtered through a 0.22 μm filter. EVs were isolated and purified from 200 mL of the filtered cell culture supernatant using the four methods described below. The solutions containing the purified EVs were subsequently analyzed using Nanoparticle Tracking Analysis (NTA) and ELISA.
[Isolation and purification method]
- MassivEV™
- MassivEV™ EV Purification Column PS / MassivEV™ Purification Buffer Set (This product, PS affinity method)
- TFF
- Only Tangential flow filtration (500 kDa)
- TFF+AEX
- Tangential flow filtration (500 kDa)+Anion-exchange chromatography
- TFF+SEC
- Tangential flow filtration (500 kDa)+Size-exclusion chromatography
(1) Particle analysis by NTA
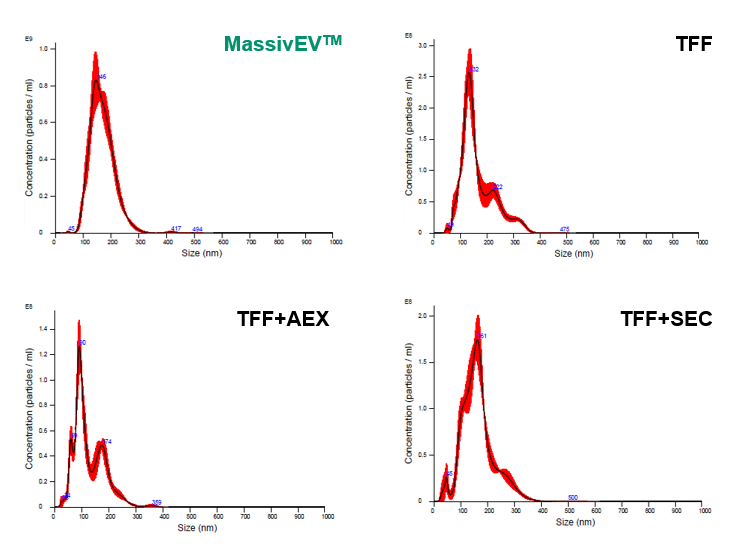
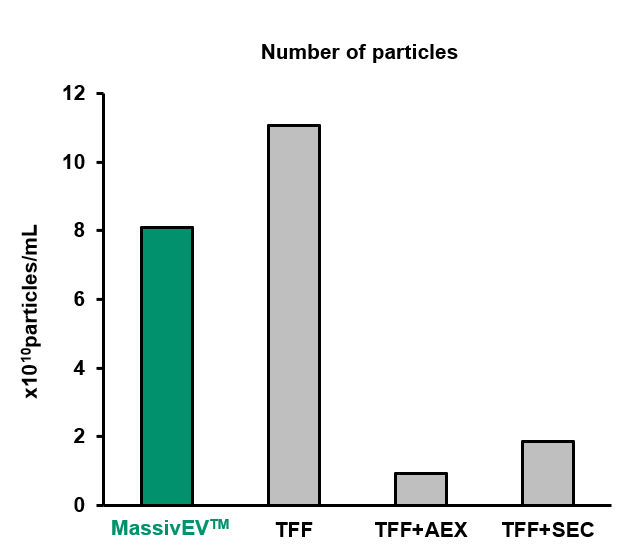
[Result]
MassivEV™ yielded a greater number of particles compared to TFF+AEX or TFF+SEC, but fewer particles were obtained compared to TFF alone.
(2) Comparison of EV recovery as measured by CD63 ELISA and CD81 ELISA (n=3)
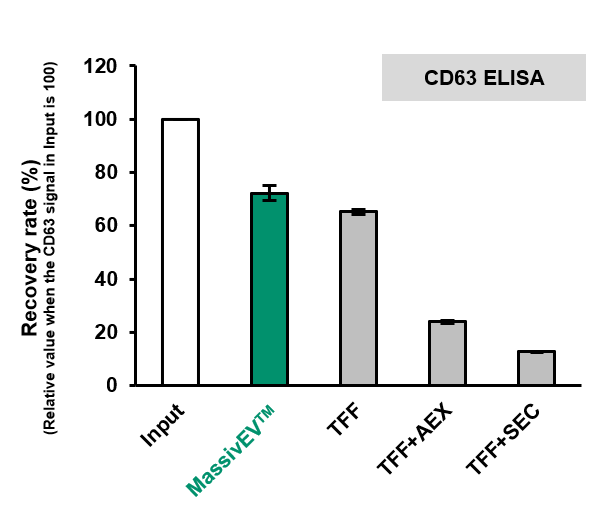
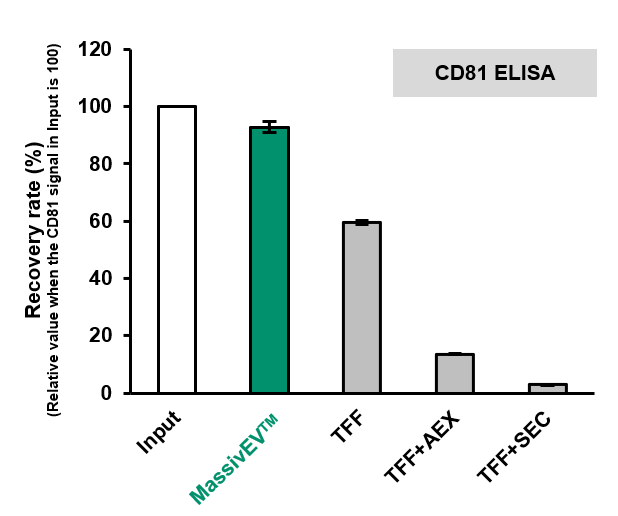
[Result]
MassivEV™ achieved a higher rate of recovery of EVs compared to conventional methods.
(3) Number of particles per protein
The ratio of protein:particle is a useful indicator of EV purity6). The total protein content in the collected EV solution was measured using the BCA method, and the particle count was determined by NTA. The number of particles per 1 μg of protein was then compared.
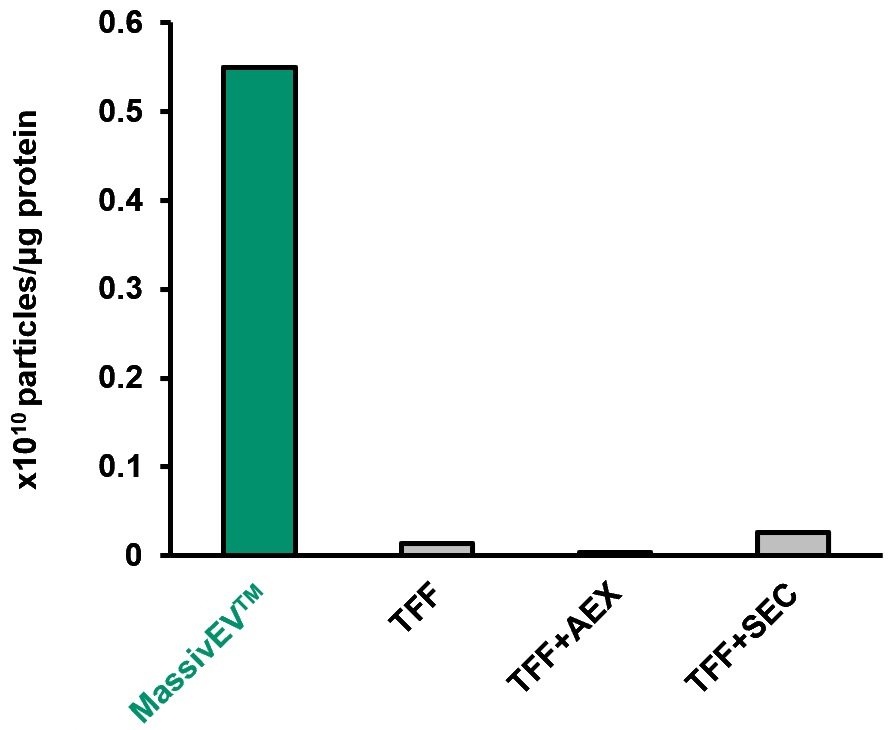
[Result]
Using MassivEV™ resulted in a higher number of particles per 1 μg of protein compared to traditional methods, indicating that EVs with higher purity can be recovered.
About commercial or for-profit purposes
Please use this product for research purposes only. If you wish to use it for commercial or for-profit purposes, please contact Fujifilm Wako at labchem_wkeu@fujifilm.com.
References
- Phinney, D. G. and Pittenger, M. F.: Stem cells, 35(4), 851(2017).
Concise review: MSC-derived exosomes for cell-free therapy - Nakai, W. et al.: Sci. Rep. 6(1), 1(2016).
A novel affinity-based method for the isolation of highly purified extracellular vesicles - Mansouri, N. et al.: JCI insight, 4(21), e128060(2019).
Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes - Angioni, R. et al.: Int. J. Mol. Sci., 21(22), 8874(2020).
Administration of human MSC-derived extracellular vesicles for the treatment of primary sclerosing cholangitis: preclinical data in MDR2 knockout mice - Perets, N. et al.: Mol. Autism, 11(1), 65(2020).
Exosomes derived from mesenchymal stem cells improved core symptoms of genetically modified mouse model of autism Shank3B - Théry, C. et al.: J. Extracell. Vesicles, 7(1), 1535750(2018).
Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.




