Antibody Optimization Service with aiProtein®

This service optimizes antibody properties, including protein yield (host expression levels), thermal/structural stability, solubility and more at little cost of antigen-binding potencies (affinity). The optimization is powered by RevolKa's advanced artificial-intelligence (AI)-driven protein engineering platform, aiProtein®. This innovative AI technology designs high-performance antibodies with a high probability of success. This service also offers multi-property optimization and supports a wide range of antibody modalities, including monoclonal IgG, scFv, and VHH can be optimized. Antibody humanization, as well as affinity recovery after humanization is also available.
Antibody Optimization is Available for the Following Properties:
- Improved stability
- Increased expression levels
- Improved solubility
- Affinity recovery after humanization
- Other physicochemical properties (subject to consultation)
Service and Deliverables
- A preliminary technical consultation will be placed before starting a project to share customer’s antibody information and properties of interest.
- The lead time from the submission of the customer’s antibody protein sequence to the delivery of optimized antibody sequences is 8 to 11 months depending on requirements in experiments, such as protein expression system.
- The deliverables include approximately 5 protein sequences of optimized variants and experimental data regarding improved properties. All of the variants will be experimentally validated by RevolKa.
Details of Antibody Optimization Service
Training Data generation for AI engineering
- Customers are to share the protein sequence information of Customer’s antibody
- RevolKa will design antibody variants for AI training.
- RevolKa will collect training data of the designed variants at RevolKa’s lab.
AI-driven design of optimized antibodies
- RevolKa’s AI engine will be trained with the prepared training data to build a customer’s antibody specific AI machine.
- The AI machine will generate candidate variants sequences, those of which will be expressed and purified by RevolKa to examine the properties of interest, including affinity. Please note that the antigen protein will be requested to be provided by customers in case that the antigen is not commercially available.
Service Flow
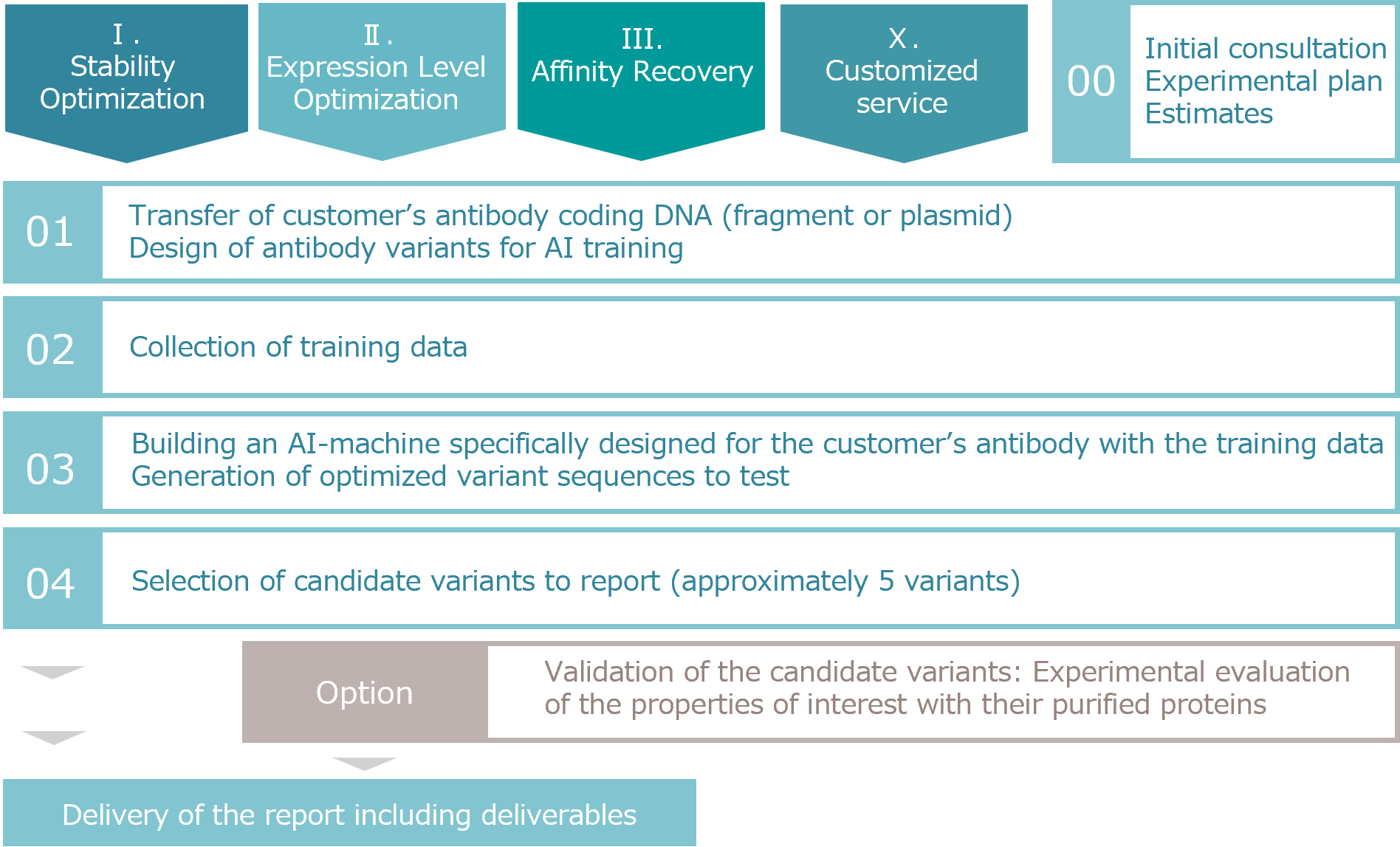
Case Studies
Case Study(1) : Improved Expression and Stability of nivolumab
Improved Stability
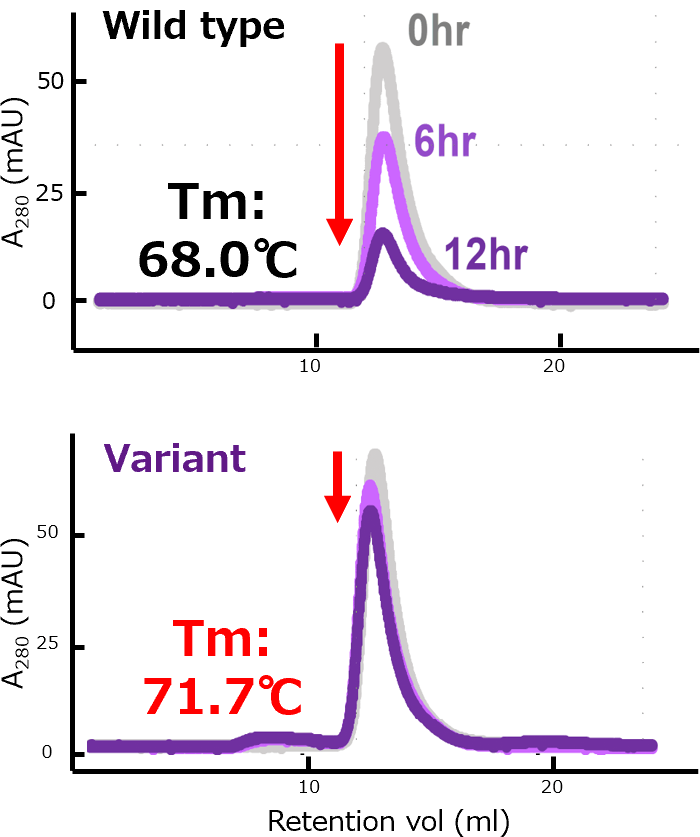
Thermal stability of nivolumab and an aiProtein®-optimized variant were tested at 60 °C for 0, 6, and 12 hours. Size-exclusion chromatography (SEC) analysis demonstrated extremely high stability of the aiProtein® variant.
Increased Expression Levels
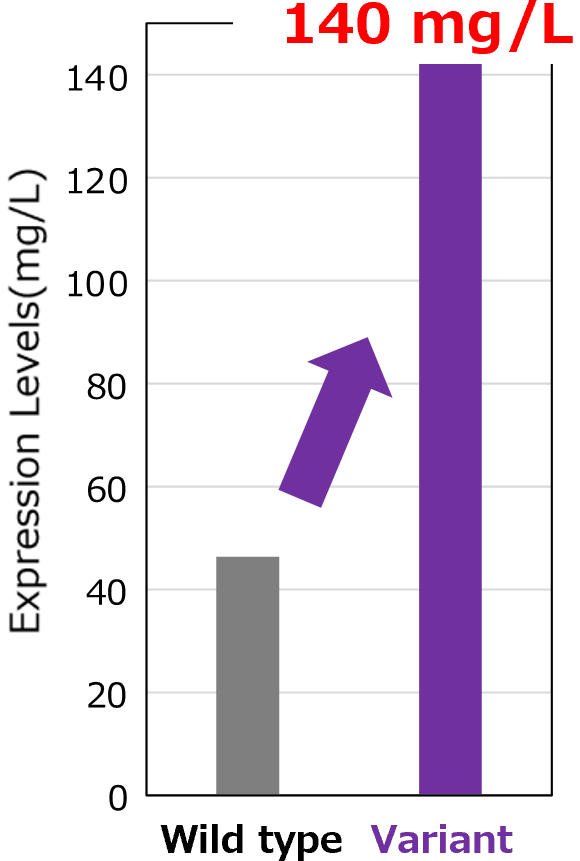
The nivolumab variant achieved a yield of 140 mg/mL (3-fold higher than nivolumab) in a transient Expi293F mammalian cell secretion system.
Protected Affinity
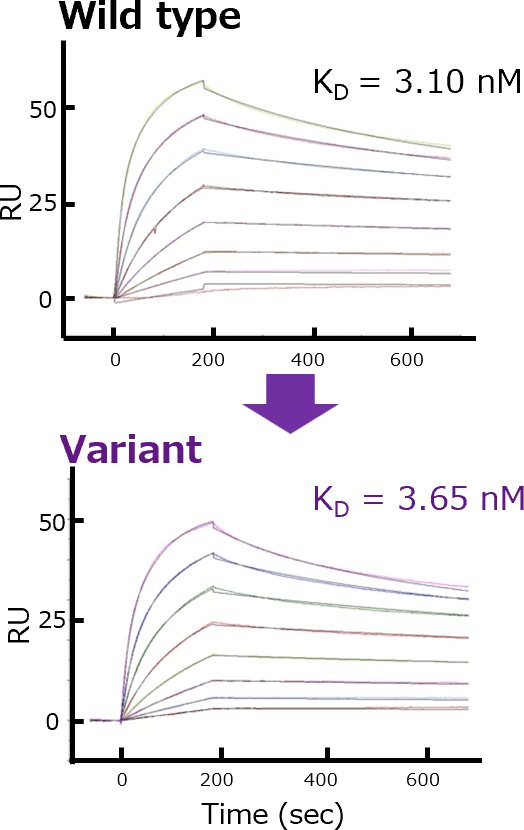
Nivolumab and the variant showed a comparable antigen binding and dissociation constant values in a surface plasmon resonance analysis.
Case Study(2) : Improved Expression of Difficult-to-express scFv
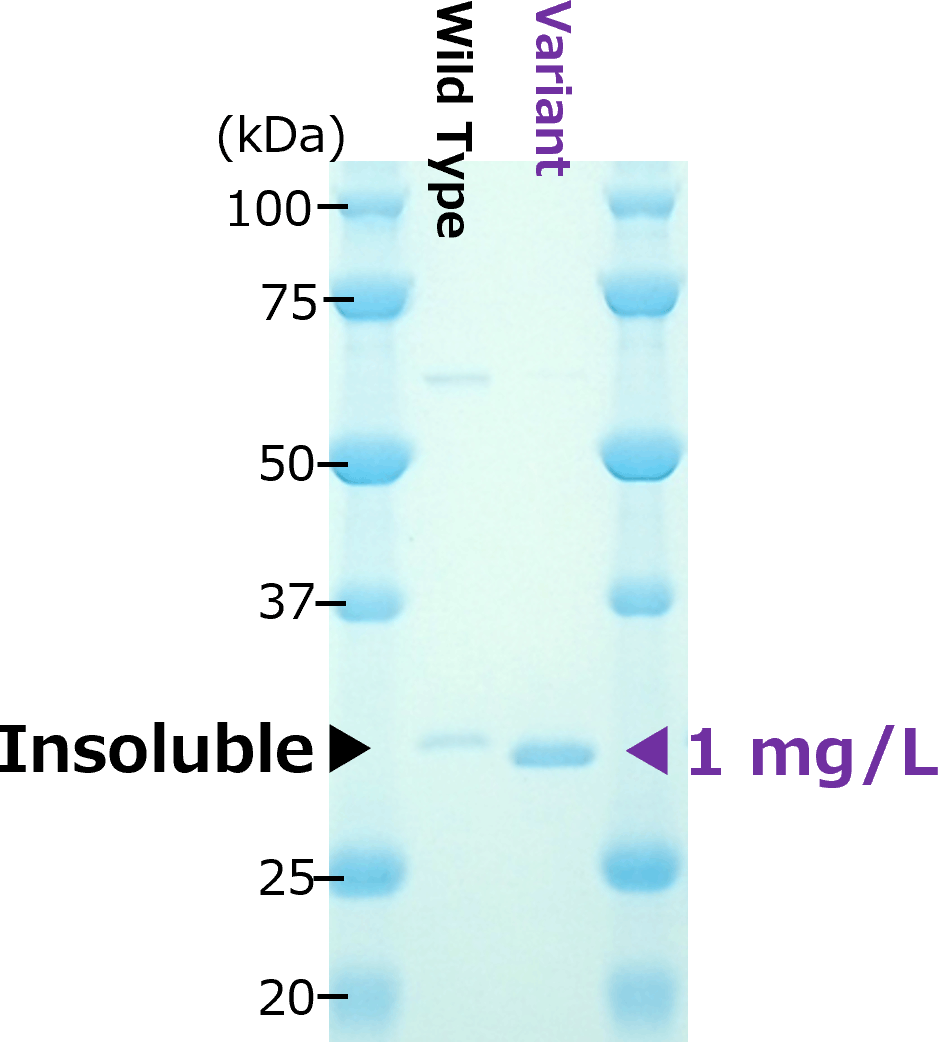
Two examples of scFv (left:scFv-A、right:scFv-B)
scFv-A
scFv-B
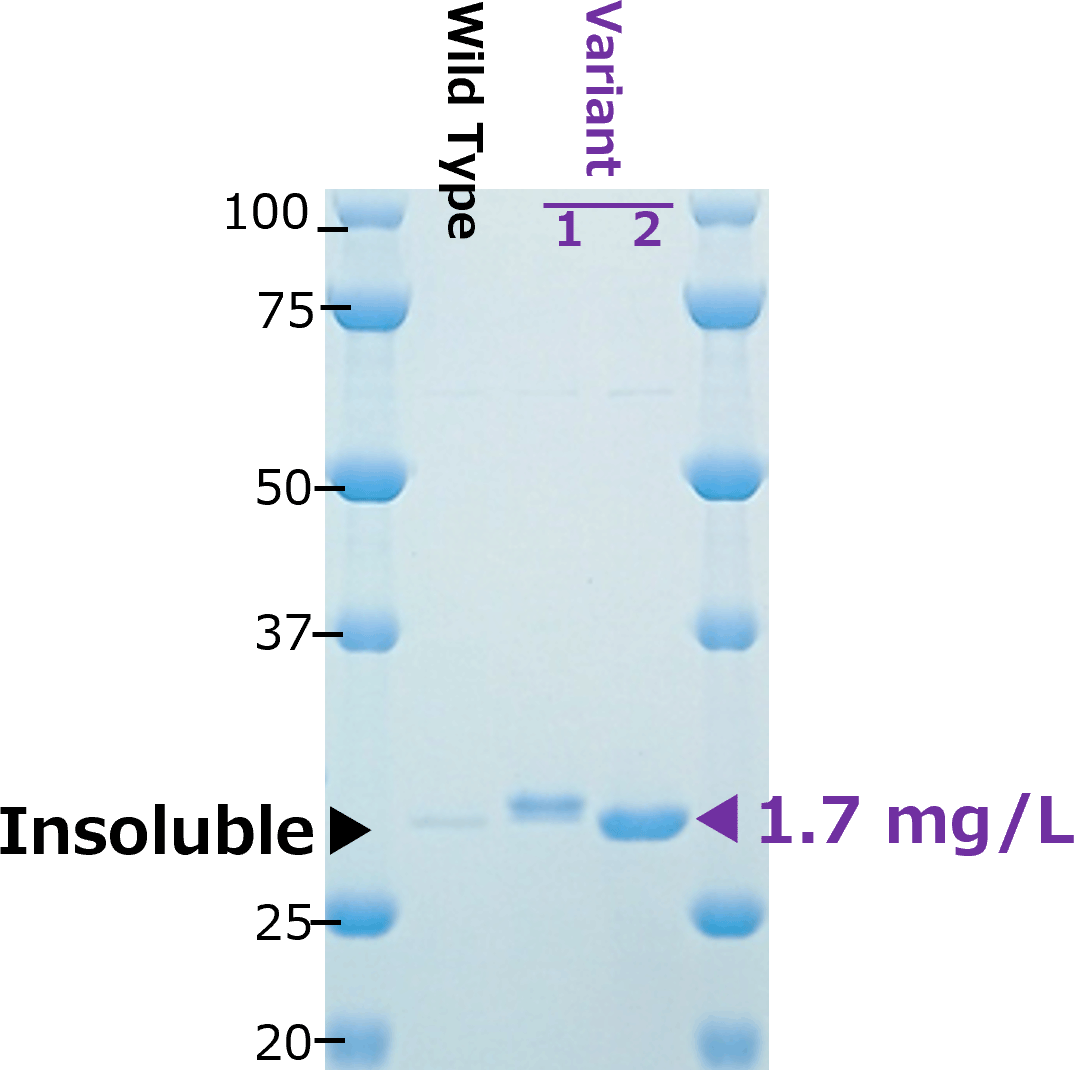
Two difficult-to-express single-chain variable fragments (scFvs): scFv-A (left) and scFv-B (right), were optimized by aiProtein® to improve yields in E. coli BL21(DE3). The scFv-A and scFv-B variants generated by aiProtein®(scFv-A: 1 variant, scFv-B: 2 variants) exhibited a significant increase in yields. Binding affinity to the antigen of the two scFv-B variants was comparable to that of the wild-type. Affinity of the scFv-A variant was not tested.
Case Study(3) : Improved Solubility and Yield of nivolumab
Enhanced Solubility
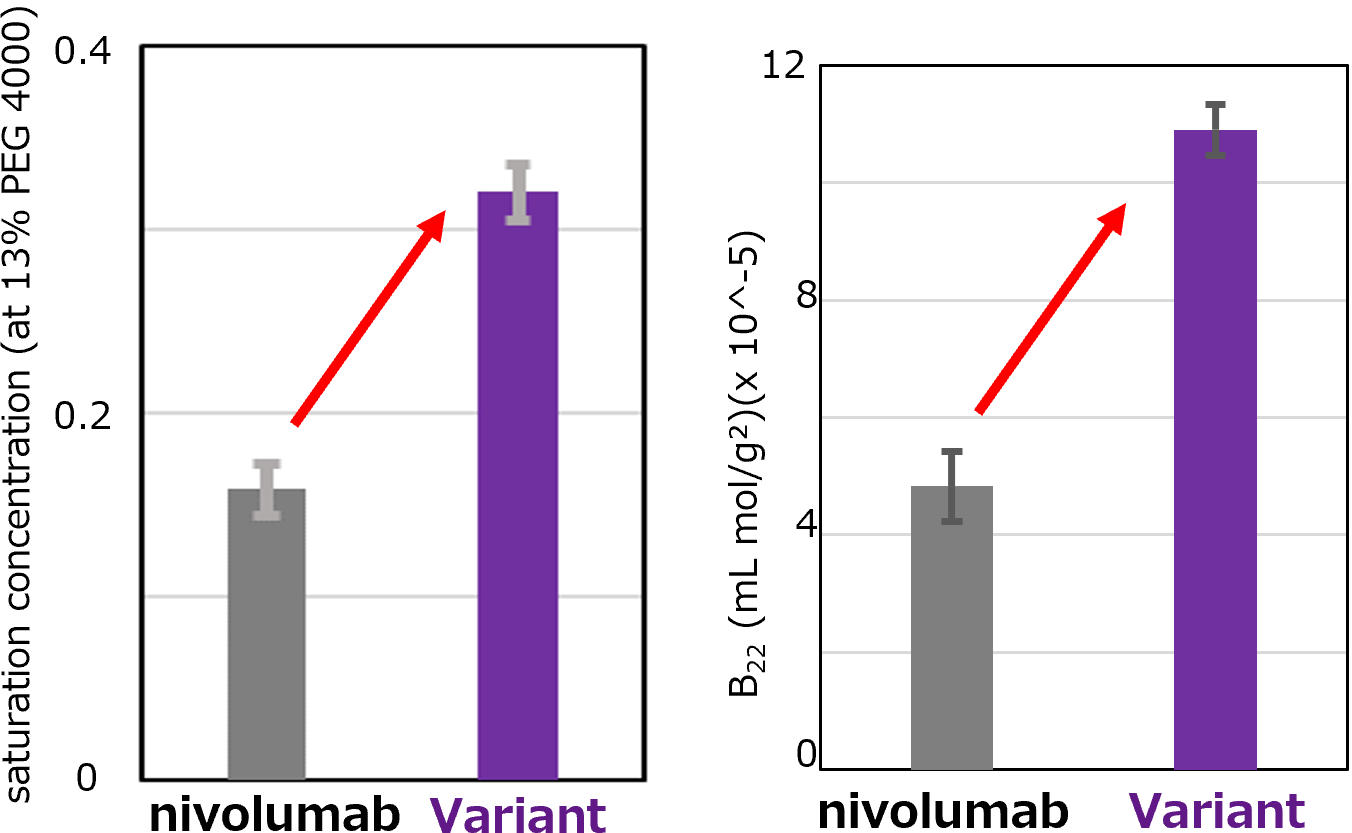
Solubility of monoclonal antibody was tested by a polyethylene glycol (PEG) method. A nivolumab variant generated by aiProtein® showed higher saturation concentration under a 13% PEG condition compared to nivolumab (left). Consistent with this result, second virial coefficient(B22), representing protein colloidal stability, was also higher for the variant than that of wild-type (right), suggesting that the variant can be potentially formulated at a high concentration. The binding affinity of the variant was comparable to that of wild-type.
Increased Expression Levels
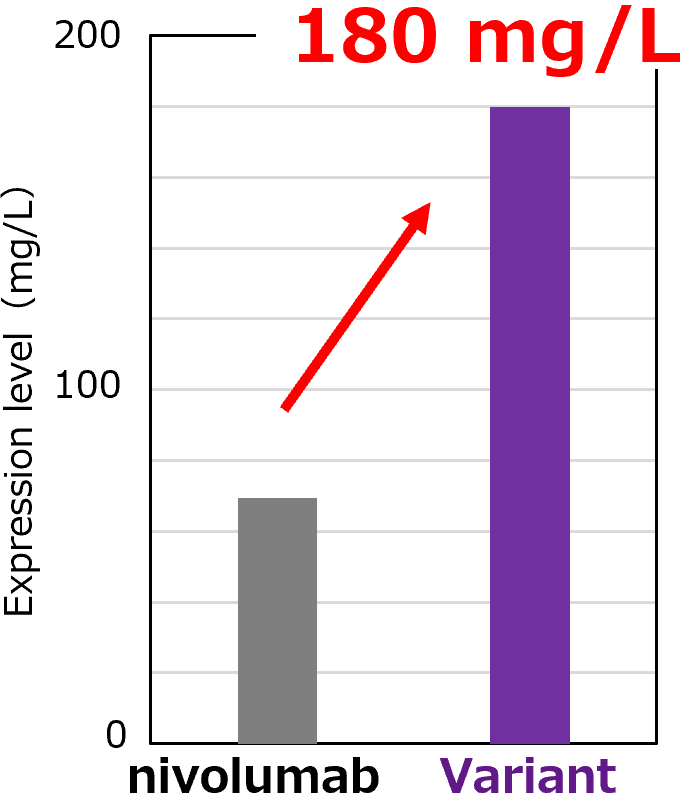
The nivolumab variant antibody achieved a yield of 180 mg/L (3-fold higher than nivolumab) in a transient Expi293F mammalian cell secretion system.
Case Study(4) : Improved Expression and Stability of humanized VHH
Increased Expression levels
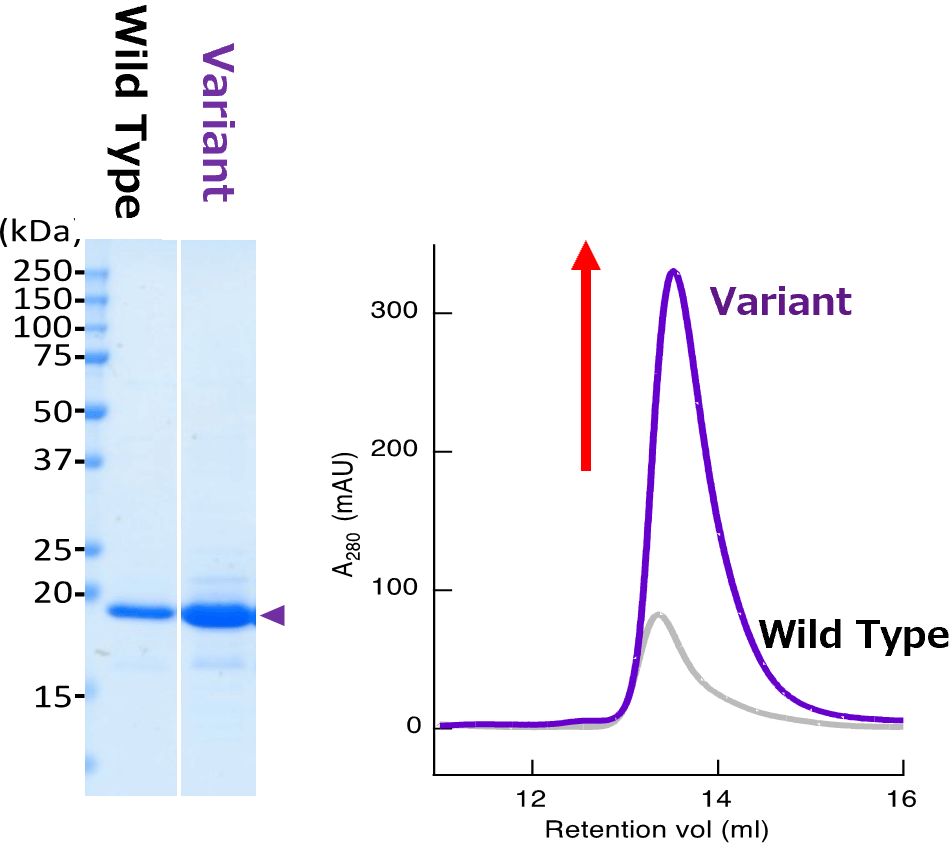
A variable heavy domain of heavy chain (VHH) that showed severe aggregation after humanization was optimized by aiProtein® to improve yields in E.coli BL21(DE3) due to aggregation issues. The variant VHH exhibited a significant increase in yields (Left) and monodispersity in size-exclusion chromatography analysis.
Improved Stability
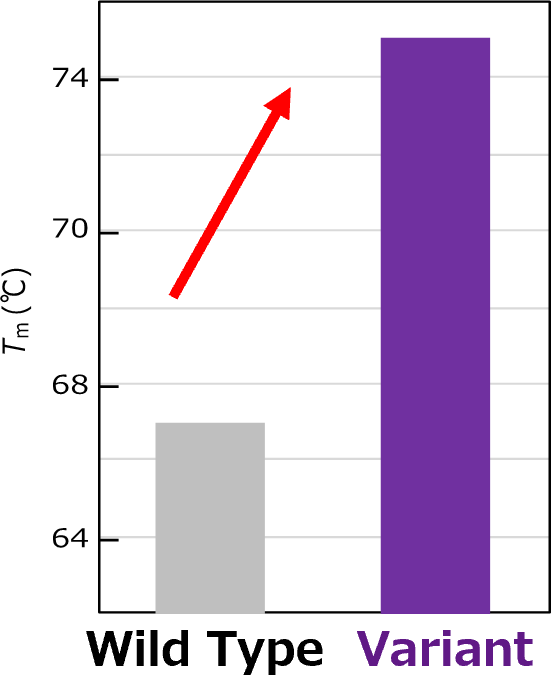
Denaturation temperature (Tm), representing structural stability of the protein, was measured using a thermal shift assay. The variant VHH generated by aiProtein® exhibited a significant increase in thermal stability of 6 °C.
Case Study(5) : Improved Expression and Stability of Diabody
Increased Expression Levels
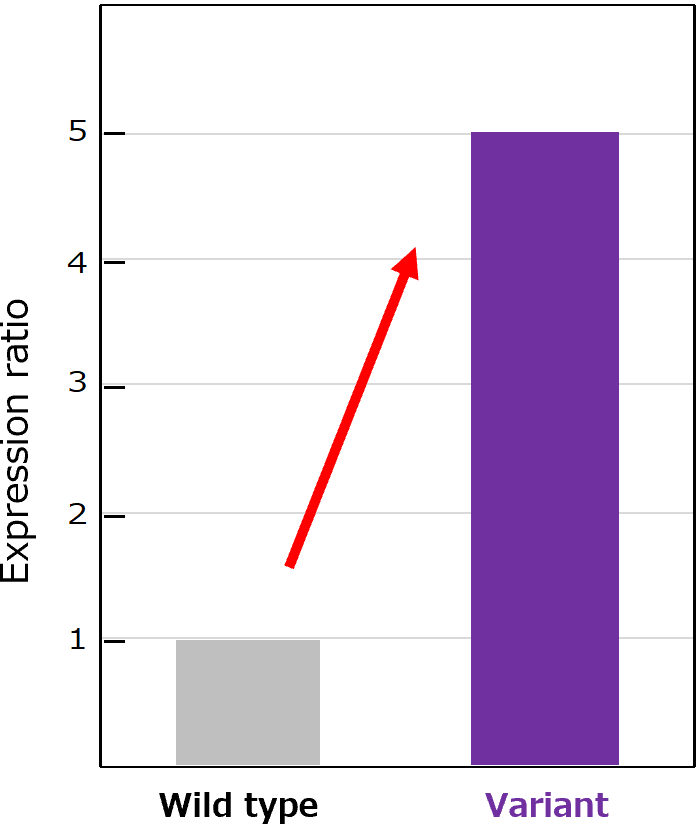
A difficult-to-express diabody was optimized by aiProtein® to improve yields in E. coli BL21(DE3). Quantitative SDS-PAGE analysis showed a 5-fold increase in yield for the aiProtein®-generated variant.
Enhanced Stability
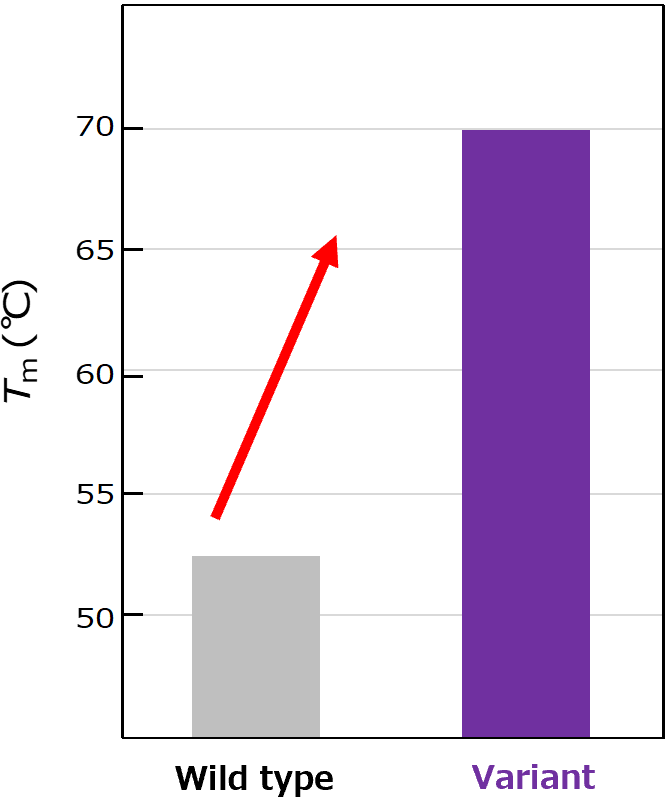
Denaturation temperature (Tm), representing structural stability of the protein, was measured using a differential scanning calorimetry assay. The variant VHH generated by aiProtein® exhibited a significant increase in thermal stability of more than 15 °C.
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



