GMP-compliant Small Molecules
It has been reported that small molecules play a critical role in maintaining the undifferentiated state and inducing the differentiation of ES/iPS cells.
We offer small molecules manufactured in compliance with ICH-Q7 (GMP for APIs). These products are produced under GMP and are designed for use as raw materials in the commercial production of regenerative medicines. They are well-suited for use in the manufacturing processes of regenerative medicines utilizing human ES/iPS cells and other stem cells.
Products Lineup
What does GMP compliant mean for our small molecules?
Our GMP-compliant small molecules are managed in accordance with ICH-Q7 (GMP for APIs) as follows.
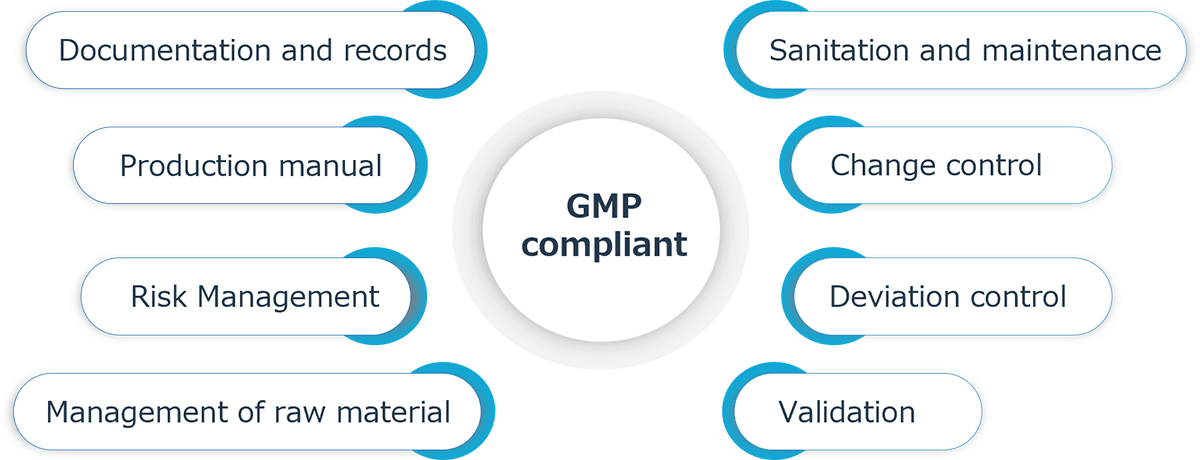
Feature
- GMP compliant
- Process validation and analytical validation carried out
- No animal-derived ingredients are used in the raw materials
- Conduct the following quality tests
- Assay
- Clarity and color solution
- Residual solvent
- Residual metal
- Viable count test
- Bacterial endotoxins test
- Mycoplasma test
*Test items vary depending on the product, so please check each product specifications.
Small Molecules Comparison Chart
In addition to GMP-compliant products, we offer a wide range of other products.
Please select the product that best suits your application, such as commercial production or research.
| ICH-Q7 | ISO9001 | Sterility test | Viable count test | No animal-derived ingredients used | Bacterial endotoxins test | Mycoplasma test | Residual solvent test | Powder/ Liquid | |
|---|---|---|---|---|---|---|---|---|---|
| GMP-compliant products | - | Powder | |||||||
| MF products | - | - | - | Powder | |||||
| CultureSure™ Series (Powder) products | - | - | - | - | Powder | ||||
| CultureSure™ Series (Liquid) products | - | - | - | Liquid |
*Test items vary depending on the product, so please check the product specifications.
Glossary
GMP
Good Manufacturing Practice (GMP) is a standard for manufacturing and quality control of pharmaceutical drugs, etc., and should be observed by manufacturers of pharmaceutical drugs, etc.
The GMP standards are based on the following three principles:
- Minimizing human errors during manufacture
- Preventing contamination and quality deterioration of pharmaceutical products
- Designing a system for high quality assurance
ICH-Q7 (GMP for APIs)
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an international initiative to create guidelines on pharmaceutical regulations from scientific and technical perspectives by joint efforts of regulatory authorities and representatives of the pharmaceutical industry.
Among these guidelines, ICH-Q7 (GMP for APIs (Active Pharmaceutical Ingredients)) outlines the standard practices for manufacturing management and quality control related to active pharmaceutical ingredients.
ISO9001
ISO stands for the International Organization for Standardization, a non-governmental organization. The standards established by the ISO are referred to as ISO standards. These are internationally agreed-upon criteria established by experts, covering various areas such as product manufacturing, process management, service provision, and material supply.
ISO 9001 is a standard set out for quality management systems. Many companies and organizations are certified to ISO 9001 to ensure that their customers consistently receive good quality products and services.
Product List
- Open All
- Close All
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.






