News
FeaturedHOT!
Pharma Manufacturing & QC
FUJIFILM Wako Pure Chemical to Launch "RiboNAT™ Rapid Sterility Test" to Accelerate Safety Testing for Cell Therapies
OSAKA, Japan, September 16, 2025 - FUJIFILM Wako Pure Chemical Corporation (hereafter: FUJIFILM Wako) will launch the "RiboNAT™ Rapid Sterility Test" (hereafter: RiboNAT™), a kit designed to enable rapid sterility testing for cell therapy, on September 16, 2025.
In the safety testing of parenteral drugs, the compendial sterility tests require a 14-day cultivation period*1. However, certain types of cell therapies must be administered to patients within just a few days after production, making it difficult to complete sterility testing before administration. This has led to a growing demand for sterility tests that yield results more rapidly to enhance patient safety. Additionally, for pharmaceuticals with longer shelf life, there is an added emphasis on improving efficiency by expediting sterility tests during the production phase to streamline manufacturing processes.
RiboNAT™ is a rapid sterility testing kit utilizing the Nucleic Acid Amplification Test (NAT) method*2. It delivers results in as little as seven hours, significantly reducing testing time compared to conventional sterility testing methods. Unlike standard NAT methods, which detect genomic DNA, RiboNAT™ targets ribosomal RNA (rRNA). Because rRNA exists in higher relative quantities*3 within microorganisms, this approach allows for more sensitive detection. Additionally, while conventional NAT methods can produce false positives due to residual DNA derived from dead microorganisms, RiboNAT™ minimizes this risk by inactivating residual DNA and focusing on rRNA detection.
RiboNAT™ comprises three kits: the RNA Isolation Kit 1, RNA Isolation Kit 2, and Detection Kit. Together, these kits enable a seamless process of lysing microorganisms, RNA purification, and detection using reverse transcription real-time PCR*4. Furthermore, the kit is capable of detecting bacteria and fungi, including the six strains specified in the pharmacopoeias, the quality standards for pharmaceuticals, in a single assay. Notably, for the six pharmacopoeia-listed strains, high-sensitivity detection can be achieved at levels as low as 9 CFU/mL.
As the newest addition to FUJIFILM Wako's lineup of pharmaceutical safety testing products, RiboNAT™ follows its endotoxin detection reagents and pyrogen detection kit. With commitment to the future of science, FUJIFILM Wako is dedicated to developing products that contribute to the happiness of all people. The company remains dedicated to developing and providing highly functional and high-quality products to address the evolving needs of society, supporting progress in industry and academic research in a wide range of fields, including pharmaceuticals.
*1 In the Japanese Pharmacopoeia 4.06 Sterility Tests, a 14-day incubation period is required. Regarding rapid test methods, the Japanese Pharmacopoeia General Information
*2 A technology that specifically amplifies genetic material such as DNA or RNA from minute quantities, enabling the highly sensitive and accurate detection of microorganisms and viruses.
*3 The quantity of a substance, expressed in terms of mass, volume, or number, indicating how much of the substance is present within microorganisms.
*4 A technique in which RNA is first converted into complementary DNA (cDNA) through reverse transcription, followed by specific amplification using PCR. During the process, fluorescent dyes or probes are used to measure the increase in DNA quantity in real time. This method enables rapid and highly sensitive detection of specific RNA.
Test Workflow
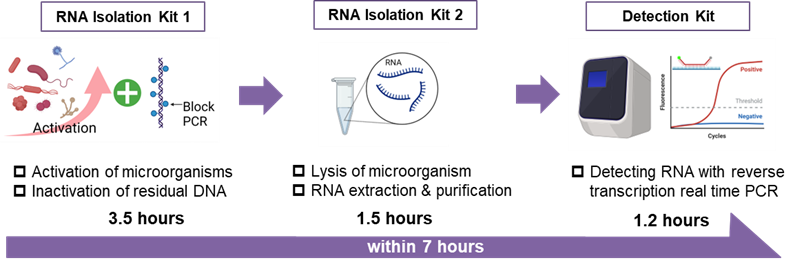
*Illustrations created using BioRender.
Product Features
- One day rapid sterility test (Approx. 7 hours).
- Detecting rRNA using RT-rt PCR and higher sensitivity than gDNA detection (9 CFU/mL).
- Reduction of false positives caused by dead microorganisms and residual DNA.
- Wide detection range of bacteria (aerobic & anaerobic) and fungi in a single assay.
Product Image
| RNA Isolation Kit 1 | RNA Isolation Kit 2 | Detection Kit |
|---|---|---|
 |
 |
 |
For inquiries on information in this media release, contact
| Media Contact | FUJIFILM Wako Pure Chemical Corporation Corporate Planning Department |
TEL: +81-50-3514-4826 |
| Customer Contact | FUJIFILM Biosciences | Email: supportfisilssupport@fujifilm.com |



