Drug Discovery Service : Assessment of Proarrhythmic Potential
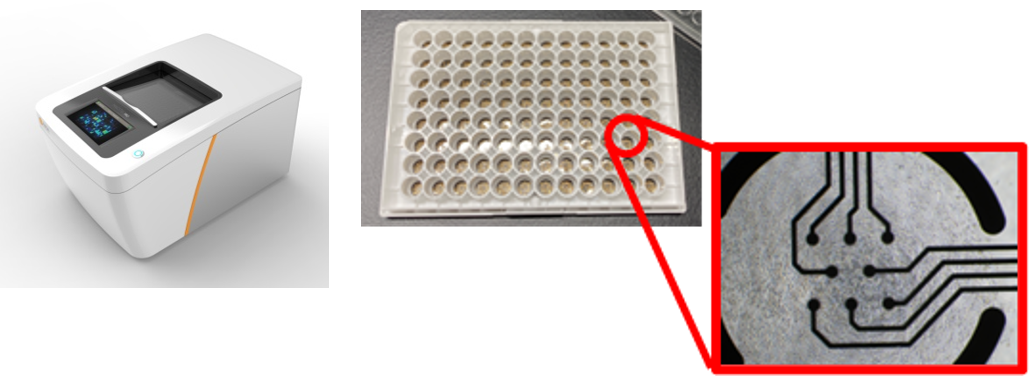
Human iPSC-derived cardiomyocytes (hiPSC-CM) have proved to be an invaluable tool for predicting the proarrhythmic potential of drug candidates in vitro. FUJIFILM is able to provide in vitro cardiotoxicity and safety pharmacology services using CiPA-validated iCell® Cardiomyocytes2, as per ICH Guidelines (S7B/E14), in addition to GLP-compliant studies. Our capabilities span from rigorous GLP-compliant studies suitable for regulatory submission to high-throughput multi-electrode array (MEA) assays in a 96-well format tailored for the drug discovery phase with ion channel blockade through alterations in extracellular field potentials.
This assay enables us to reduce the risk of prolonged drug discovery timelines and increased project costs by minimizing the dropout of promising compounds due to false positives or unforeseen cardiovascular risks detected during clinical trials. We launched this assay service in April 2023 and, as of August 2025, have evaluated 277 test substances from a diverse range of clients, including global mega-pharmaceutical companies, biotechnology startups, and academic institutions.
iCell® Cardiomyocytes2
iCell® Cardiomyocytes2 are high-purity cardiomyocytes derived from human-induced pluripotent stem cells (hiPSCs) produced by FUJIFILM Cellular Dynamics, Inc. with proprietary differentiation technologies. They recapitulate functions of native human cardiomyocytes. With over 800 peer-reviewed publications, iCell® Cardiomyocytes2 are a valuable tool for pharmacology, safety pharmacology and basic research across multiple assay platforms. Atrial, nodal, and ventricular cardiomyocytes with spontaneous electrical activity possess typical biochemical, electrophysiological, and mechanical properties of native cardiomyocytes, as well as higher expression levels of critical genes, including ion-channels, as compared to hiPSC-derived cardiomyocytes from other sources.
Basic Property as Cardiomyocytes
- High purity of cTNT positive cells1
- Sarcomere structure1
- Cardiac action potential1
- Expression of major cardiac ion channels (NaV1.5, CaV1.2, hERG) comparable to human heart2
High-Quality Extracellular Field Potential to Assess Proarrhythmic Potential
The extracellular field potential of hiPSC-cardiomyocytes is a validated biomarker for assessing the proarrhythmic potential of drugs. It can be obtained using a multielectrode array (MEA) system3, 6. iCell® Cardiomyocytes is one of the cells used for international validation studies5, 6.
<Comparison across hiPSC-CM assay, hERG inhibition and clinical QT prolongation>
| Compound | Cinical QT prolongation | hiPSC-CM assay | hERG inhibition |
|---|---|---|---|
| E-4031 | Positive | Positive | Positive |
| Terfenadine | Positive | Positive | Positive |
| Dofetilide | Positive | Positive | Positive |
| Flecanide | Positive | Positive | Positive |
| Astemizole | Positive | Positive | Positive |
| Verapamil | Negative | Negative | Positive |
| Mexiletine | Negative | Negative | Negative |
| Loratadine | Negative | Negative | Positive |
| Aspirin | Negative | Negative | Negative |
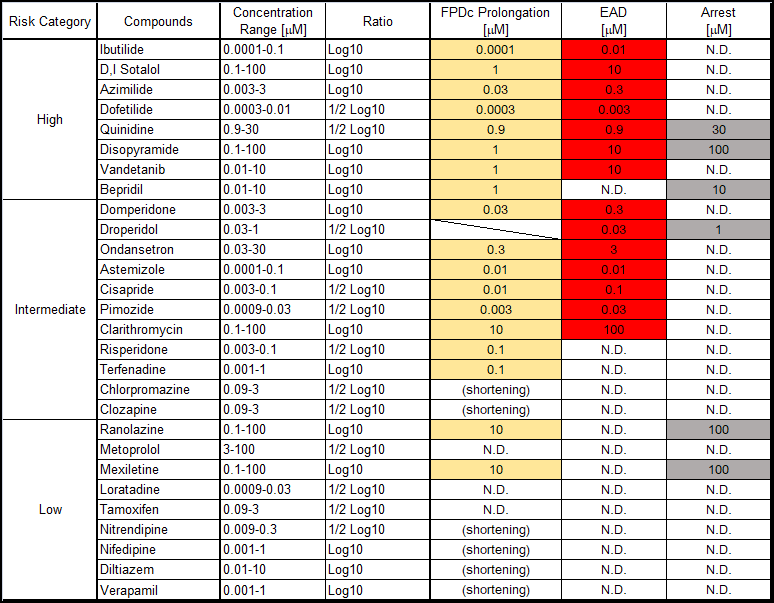
Clinical QT prolongation:Compounds with a known risk of Torsades de Pointes (TdP) in CredibleMeds® were classified as positive6.
hiPSC-CM:Compounds that prolonged the corrected field potential duration (FPDcF) by 10 % or more at a concentration below 10 mM were considered positive.
hERG:Compounds with an IC50 for hERG channel inhibition less than 10mM were regarded as positive.
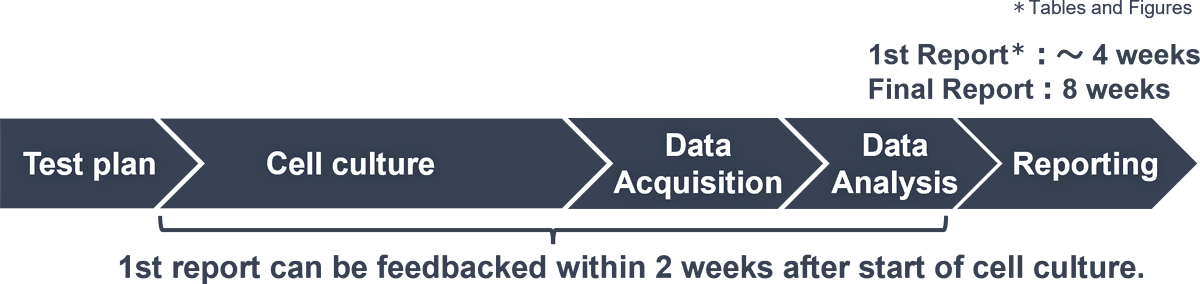
Outline of the Assay Service
Service Flow
End Points
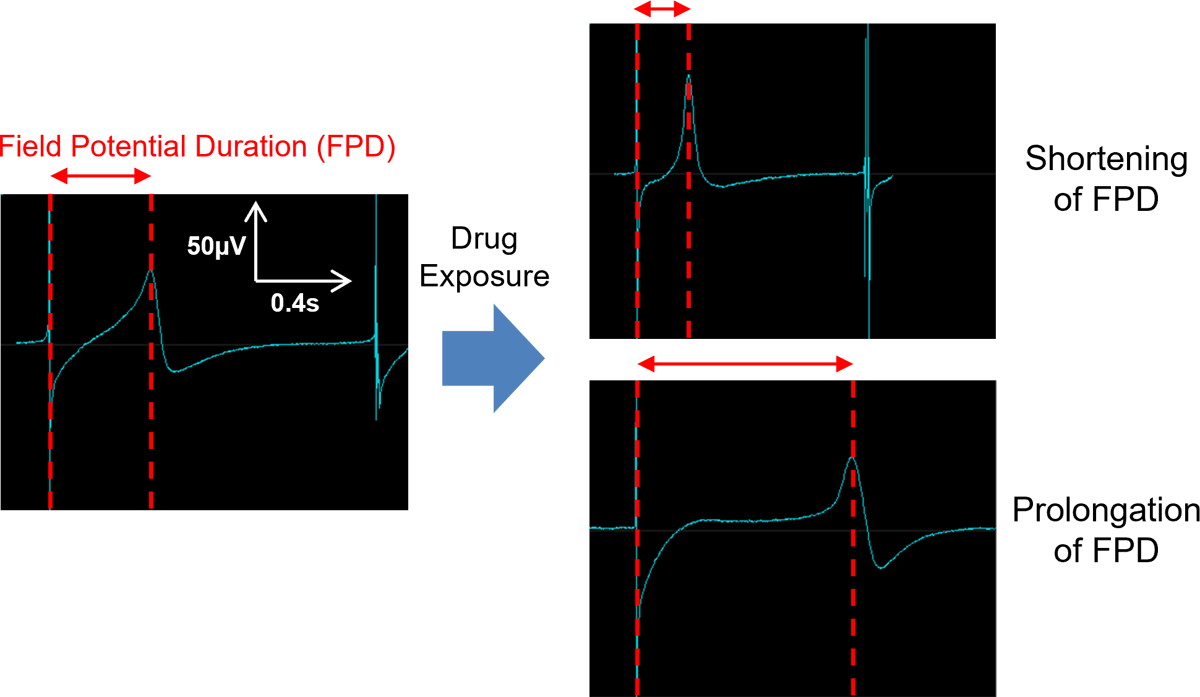
- FPDcF(FPDcF10: concentration to prolong or shorten the FPDcF by 10 %)
- Beat Rate
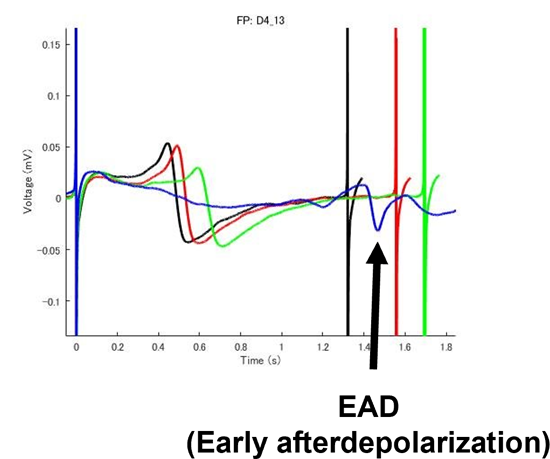
- Abnormal Wave Form(EAD:Early Afterdepolarization)
Optional
- Viability(impedance)
- 1st Peak Amplitude(µV)
- 2nd Peak Amplitude(µV)
Turnaround Time
- 1st Report (Tables and Figures) : About two weeks after initiation of cell culture
- Final Report : About 4 to 5 weeks after initiation of cell culture
Key Features
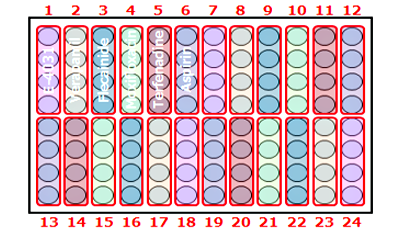
High-throughput assay ideal for screening
Our 96-well plate assay enables simultaneous evaluation of 22 compounds (n=4), boosting your screening throughput.
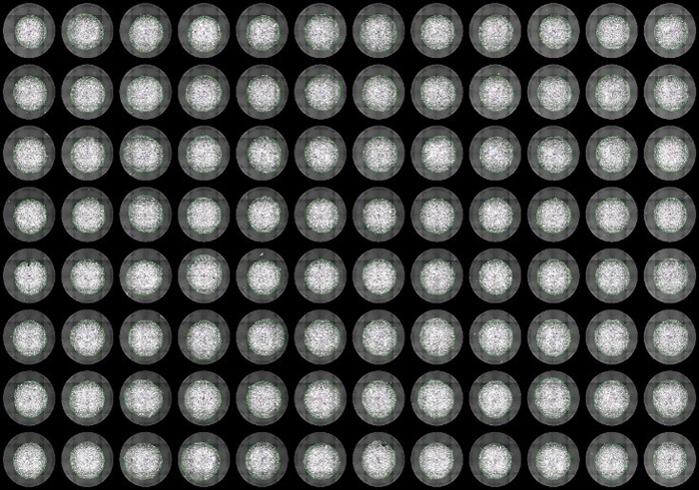
Nuclei staining [Hoechst 33342]
Our proprietary protocol ensures precise cell placement on electrodes within each well for reliable and consistent results.
Extracellular field potential
[0.1% DMSO]
Clear and analyzable signals are obtained from all wells after exposure to 0.1 % DMSO.
![Extracellular field potential [0.1% DMSO]](../../../../us/category/images/95184_img30.png)
Background Data
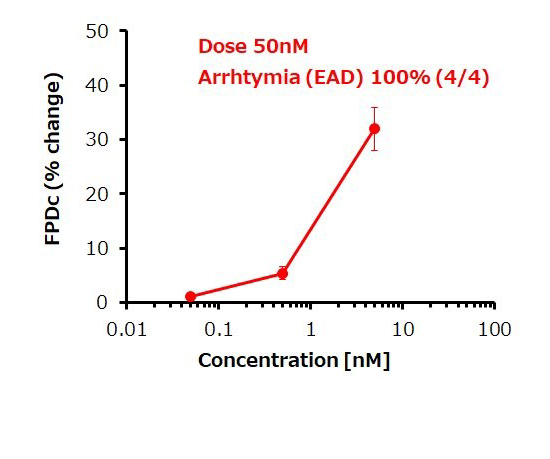
Dose response of FPDc change
E-4031 (hERG inhibitor)
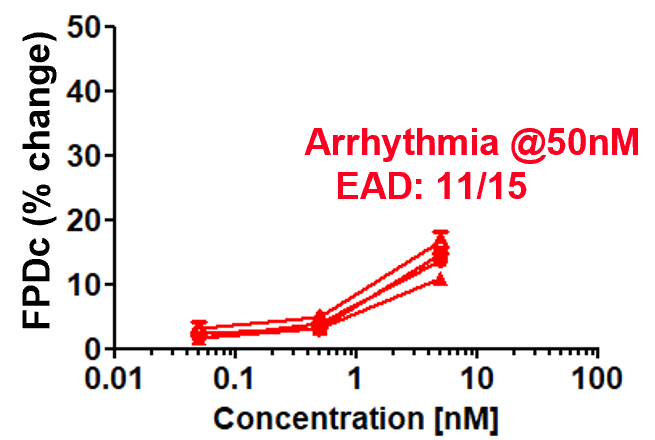
Aspirin (cyclooxygenase inhibitor)
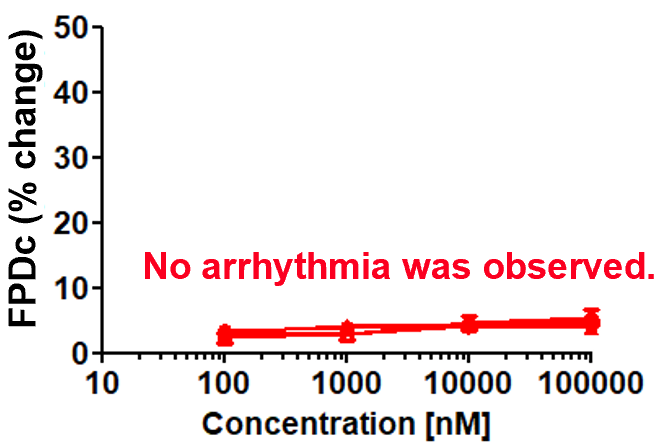
Robustness of drug response
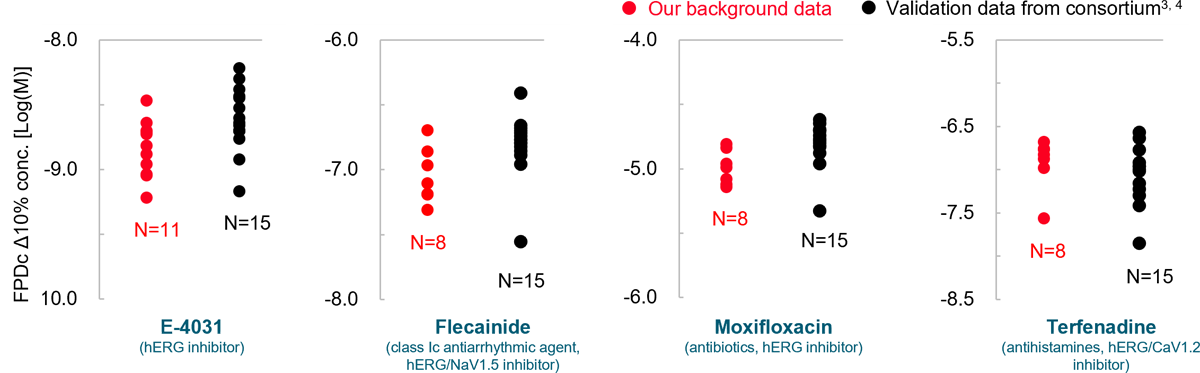
Prolongation and shortening of FPDc, as well as proarrhythmic effects, were accurately detected.
The concentration range of the effects has been verified to be consistent with previous reports.
Identification of Chronic Effects
Chronic effects of test compounds on proarrhythmic potential can be detected by long-term exposure.
Aspirin
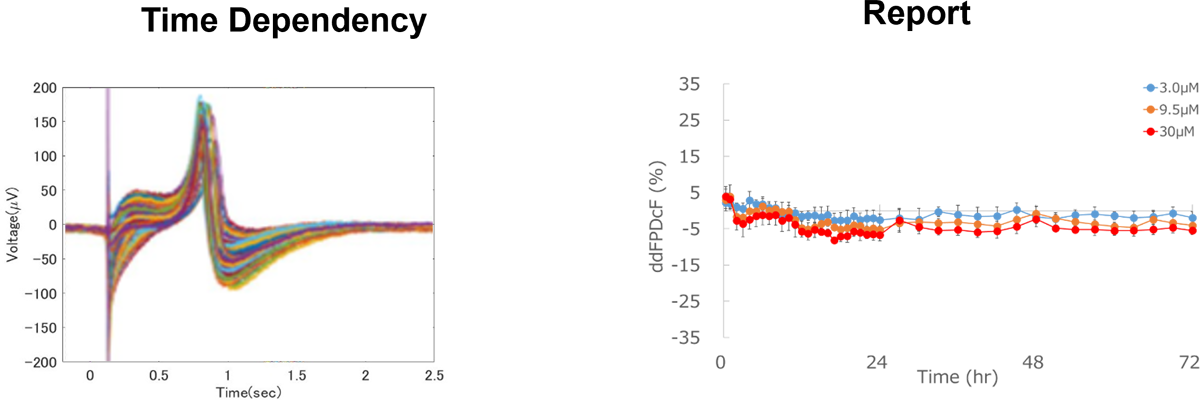
No effect
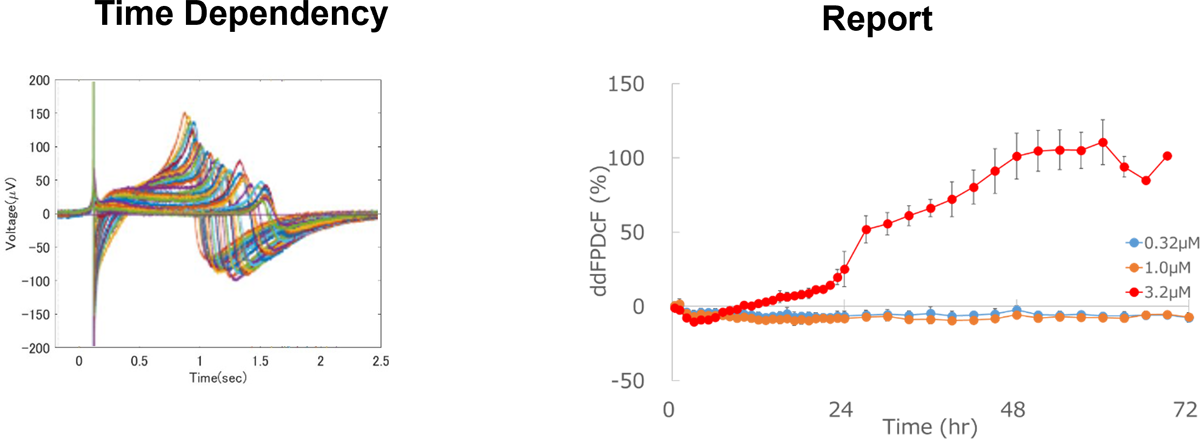
Prolongation of field potential duration
Optional services
Optional Services
- Cardiac contractility assay (evaluation of cardiomyocyte contractility)
- Pharmacology assay using cardiomyocytes (healthy and disease models), including assay development
- Identification of ion-channel inhibition from extracellular field potential recordings, including IC50 prediction.
References
- Ma et al., Am J Physiol Heart Circ Physiol 30 (2011) H2006-H2017
- Kodama et al., J Pharmacol. Sci. 140 (2019) 325-330
- Kitaguchi et al., J Pharmacological and Toxicological Methods 78 (2016) 93-102
- Nozaki et al., Regulatory Toxicology and Pharmacology 77 (2016) 75-86
- Blinova et al., Cell Reports 24 (2018) 3582-3592
- Ando et al., Journal of Pharmacological and Toxicological Methods 84 (2017) 111-127
Poster presentations
| Date | Conference | ポスター名 | |
|---|---|---|---|
 |
2025/7/2~7/4 | The 52nd Annual Meeting of the Japanese Society of Toxicology | Evaluation of the Long-Term Exposure Effects of Compounds on Human iPS Cell-Derived Cardiomyocytes Using a Multi-Electrode Array |
 |
2025/2/20~2/21 | The 16th Japanese Safety Pharmacology Society Annual Meeting | Utilizing hiPSC-Derived Cardiomyocytes for Comprehensive Electrophysiological and Inotropic Evaluation of Cardiovascular Safety in Early Drug Discovery |
 |
2024/9/22~9/25 | 2024 SPS Annual Meeting | Comprehensive Suite of Assays for Assessment of Cardiotoxicity Using Human iPSC-derived Cardiomyocytes |
 |
2024/7/3~7/5 | The 51st Annual Meeting of the Japanese Society of Toxicology | Development of cardiac contractile function evaluation system by measuring impedance of human iPScell-derived cardiomyocytes using a multipoint electrode array |
For research use or further manufacturing use only. Not for use in diagnostic procedures.
Product content may differ from the actual image due to minor specification changes etc.
If the revision of product standards and packaging standards has been made, there is a case where the actual product specifications and images are different.



