[Chromatography Q & A] What is a difference in use of acetonitrile and methanol, which are used frequently in reverse-phase HPLC analysis?
A water and organic solvents is used frequently as an eluate of reverse-phase chromatography. In particular, methanol and acetonitrile are often used as organic solvents. Methanol is cheaper than acetonitrile in price but it seems difficult to use methanol in all of the analyses.
The advantages of methanol and acetonitrile are summarized below.
Absorbance: Small with acetonitrile for HPLC
The absorbances of acetonitrile for HPLC and methanol (guaranteed reagent) are compared in Figure 1. The smaller the absorbance of an organic solvent used in the mobile phase is, the smaller the noise in UV detection becomes. Therefore, acetonitrile for HPLC is suitable for the high-sensitivity analysis in short UV wavelengths. With regard to the gradient baseline in UV detection, acetonitrile for HPLC has fewer ghost peaks and, although there are other organic solvents with a high compatibility with water, not many of them exhibit an absorbance smaller than that of acetonitrile for HPLC.
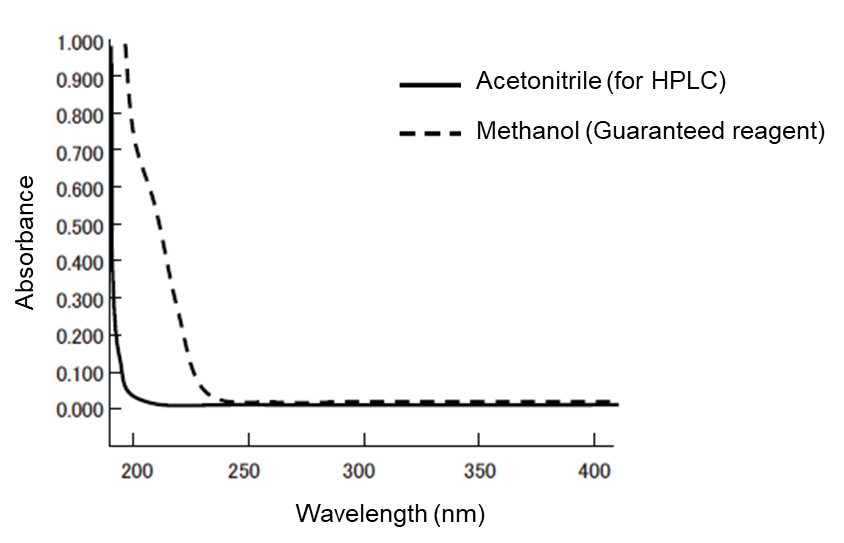
Figure 1. Absorbance spectrum of acetonitrile for HPLC and methanol (guaranteed reagent)
| Wavelength (nm) | Absorbance | |
|---|---|---|
| Methanol Guaranteed reagent | Acetonitrile for HPLC | |
| 200 | 0.7078 | 0.0235 |
| 210 | 0.4813 | 0.0137 |
| 220 | 0.1959 | 0.0077 |
| 225 | 0.0928 | 0.0051 |
Pressure: Lower with acetonitrile
Relationship of the mixing ratios of water and acetonitrile and of water and methanol to the feeding pressure are shown in Figure 2. The pressure of methanol increases by mixing with water but the pressure of acetonitrile does not increase that much. Acetonitrile eluent does not exert extra pressure on the column at the same flow rate.
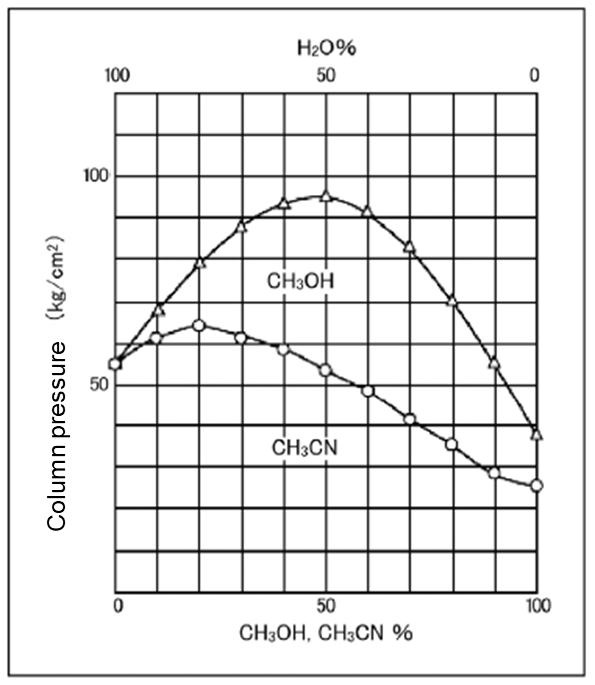
-
Column Size: 4.6 mm x 150 mm
Eluent: CH3CN-H2O/CH3OH-H2O
Flow Rate: 1.0 mL/min. at 30℃
Column: Wakopak®Wakosil-Ⅱ 5C18HG
Figure 2. Comparison of column pressure of the acetonitrile eluent and methanol eluent
Elution capacity: Acetonitrile has a higher elution capacity in general
When acetonitrile or methanol are mixed with water in the same ratio, the acetonitrile eluent exhibits a higher elution capacity than methanol eluent in general. Especially when the mixing ratio is low, the mixture with acetonitrile may take not more than a half time than that with methanol to obtain the same retention time.
Selectivity of separation (elution): Depends on the solvent
The selectivity of separation depends on the solvent. The order of elution may reverse between acetonitrile and methanol. It also differs by the mixing ratio with water. It is considered to be due to differences in chemical properties of organic solvent molecules.
Peak shape: May be different
The tailing may be greater with the acetonitrile eluent, and be suppressed with the methanol eluent. This tendency is seen with compounds such as salicylic acid (phenols with carboxyl and methoxy groups at ortho position). This is considered to be due to different way of involvement of the mobile phase in the interaction (adsorption) between the silica surface and the target component depending on the chemical properties of the organic solvent molecule. With a reverse-phase polymer column, the tailing tendency is often inconspicuous with the acetonitrile eluent.
Sample solubility: Methanol has a higher solubility than acetonitrile for ionic compounds such as acidic and basic compounds
With the samples prepared with a highly soluble solvent, it is easy to reduce the injection volume and adjust the organic solvent concentration between the mobile phase and the sample.
Deaeration of mobile phase: Caution is required for the acetonitrile eluent
Methanol generates heat by mixing with water and excess dissolved air readily comes out (deaerates) as bubbles. On the other hand, as acetonitrile absorbs heat and is cooled, bubbles generate later when its temperature returns to room temperature. The temperature of the mobile phase during deaeration must be taken into consideration.
Ionization efficiency of LC/ESI-MS: Higher with acetonitrile
While ionization of ESI produces fine droplets from the mobile phase, it is more efficient with lower solvent viscosity and, therefore, acetonitrile having the lower viscosity than methanol shows a higher ionization efficiency.
As described above, it can be said that acetonitrile for HPLC is a highly compatible organic solvent for reversed-phase HPLC. However, if the selectivity / peak shape is poor, it is considered efficient to optimize by trying HPLC with methanol. Knowing and considering the characteristics of each organic solvent to establish the optimum analytical conditions will lead to solvent saving and cost reduction.




