[Technical Report] Development of a Fully Automated Analytical Method for PFOA, PFOS, and PFHxS in Water Using an Online SPE-LC/MS System
This article was written by Ryoichi Sasano, AiSTI SCIENCE CO., Ltd., for Vol. 28, (January 2024) of ChemGrowing.
1. Introduction
Perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorohexane sulfonic acid (PFHxS) are widely used in daily life as processing aids for fluoropolymers, paints, water repellents, emulsifiers, fire extinguishing agents, and frying pans. Since these substances (collectively called "PFAS") are non-volatile and persistent, there are concerns about environmental pollution and various toxicities affecting the human body due to their long-term persistence in the environment. For this reason, regulations are rapidly being tightened in many countries. It is believed that PFAS contamination is spreading into the soil, underground water, and public water supplies, including tap water, from a range of sources including factory emissions, fire extinguishing agents, and industrial waste treatment. The Japanese Ministry of the Environment has set a provisional indicator value of 0.05 ppm or less for items requiring monitoring for the protection of human health.
Therefore, the analysis method used to determine the levels of PFAS in river water requires a large sample volume, and a method using a solid-phase extraction (SPE) column has been proposed. However, this pretreatment operation is complicated and takes a long time. In addition, because PFAS may be a component of the instruments used for pretreatment as well as in analyzers due to its favorable properties' or something similar, it is necessary to be mindful of the possibility of contamination during the analysis process. A basic precaution when using instruments is to avoid using those that incorporate Teflon (polytetrafluoroethylene) in processes involving the use of solvents, standard solutions, and samples. Furthermore, instruments should be carefully cleaned with methanol to reduce the risk of PFAS contamination on the blank before use. Since it has been pointed out that PFAS may be adsorbed on glass surfaces, it is desirable to use polypropylene (PP) vials for sample preparation, especially when conducting additive recovery tests in low concentration ranges. As described above, pretreatment work in PFAS analysis is complicated and requires caution, imposes a high workload on analysts, and increases work time. Currently in Japan, there is a shortage of analytical technicians, with the associated challenge of passing on skills, so the increased workload on analysts has become a major problem.
Here, I introduce the development of a method for analyzing PFAS in river water using an online SPE-LC/MS system that enables fully automated analysis from SPE to measurement. The advantages aimed at include a much smaller sample volume, eliminating the need for nitrogen gas purge concentration, short pretreatment time, and automation.
2. Online SPE-LC/MS system
2-1. Equipment overview
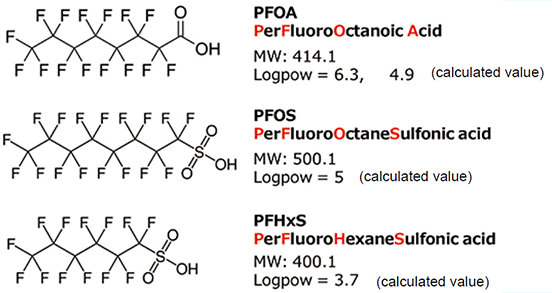
Figure 1 shows an online SPE-LC/MS system that combines an online SPE system, SPL-W100 (AiSTI SCIENCE CO., Ltd., hereinafter SPL-W100) and a liquid chromatograph-triple quadrupole mass spectrometer, LCMS-8045 (SHIMADZU CORPORATION). The online SPE-LC/MS system uses the SPL-W100 for SPE of samples and automatically injects this entire eluate into the LC for measurement by LC/MS. The online SPE-LC/MS system consists of a sample preparation section (upper part: arm-type robot) and a liquid delivery section (lower part: syringe pump and valves), and all processes from SPE to sample injection into the LC column and subsequent LC/MS measurement can be performed automatically by simply loading the sample. (Figure 2)

Figure 1. Online SPE-LC/MS system
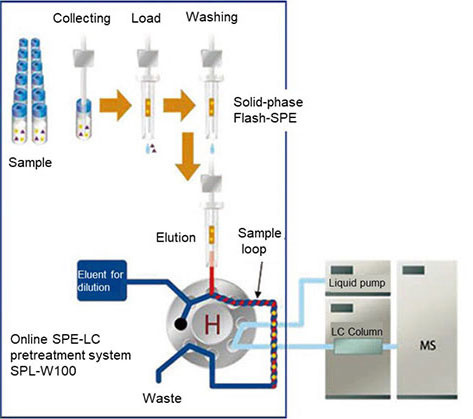
Figure 2. Conceptual diagram of online SPE-LC
The online automation of this system is made possible by Flash-SPE, a solid-phase cartridge dedicated for online SPE-LC, and MiVS (Mixing Injection Valve System), a mixing injection valve system for injecting eluate from SPE column into the LC (both patented).
The solid-phase cartridge Flash-SPE dedicated for online SPE-LC and its structure are shown in Figure 3. This solid-phase cartridge has a very compact design with a filling volume of only 2 to 5 mg, enabling elution of the target substance from the solid-phase with just several tens of µL of solvent. Furthermore, the cartridge features press-fit pipe connections on the top and bottom of the cartridge for easy connection and disconnection from the piping, resulting in a small dead space.
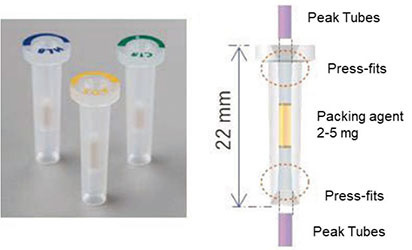
Figure 3. Flash-SPE solid-phase cartridge and its structure
In the mixing injection valve system "MiVS" (Figure 4), eluate from the solid-phase is mixed with diluent (e.g., water) in the valve and collected in the sample loop, and the entire eluate is injected into the LC column by switching the flow path. In general, the eluate from the solid-phase has a high organic solvent ratio, so by diluting it with water to reduce this ratio, the analyte is concentrated at the tip of the LC column, thus maintaining a sharp peak shape in the chromatogram.
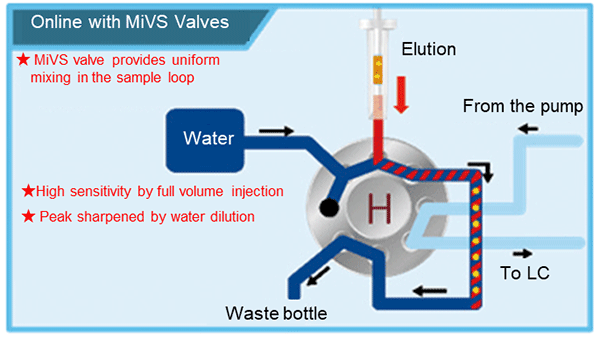
Figure 4. Mixing Injection Valve System (MiVS)
2-2. Comparison of this analysis method to the conventional method
Figure 5 shows a comparison between this analysis method and the conventional method using an online SPE-LC/MS system for the analysis of PFAS in water. In the conventional method, 1 L of sample is loaded into 500 mg of solid-phase to adsorb the analyte, the solid-phase is washed, aspirated dry and eluted with 5 mL of methanol, the eluate is concentrated with a nitrogen purge to obtain a constant volume of 1 mL, of which 1 µL is injected into the LC/MS system. In contrast, in this method, 1 mL of sample is loaded into 5 mg of Flash-SPE C18 to adsorb the analyte, the solid-phase is washed and eluted with 70 µL of methanol/water, and the entire eluate is injected into the LC column by switching the valve.
The conventional method concentrates 1 L of sample to 1 mL, but only 1 µL of that is injected into the LC column, so in effect 1 mL (1/1,000 of 1 L) is measured by LC/MS as the relative sample volume. In this method, 1 mL of sample is absorbed in the solid-phase, and the entire eluate is introduced into the LC, so the sensitivity is the same as the conventional method. By injecting the entire eluate from the solid-phase into the LC, the sample volume can be reduced to 1/1,000, and the pretreatment process can be scaled down. The pretreatment time has been reduced from 120 minutes with the conventional method to 12 minutes using this method, and full automation from pretreatment to measurement has been achieved with the dedicated online Flash-SPE and the mixing injection valve system MiVS.
There was concern that the PFOA peak shape would become broad if the entire eluate was injected directly into the LC column. Therefore, water was mixed with the eluate to lower the concentration of MeOH before LC column injection, and a sharp peak shape was obtained. In addition, compared with conventional methods, this method does not require the cleaning of pretreatment equipment because it is a closed system, and the lines through which the sample passes are always cleaned, so the risk of PFAS contamination is considered to be low.
This system allows the pre-treatment of the next sample to be carried out concurrently during the LC/MS measurement, so that pre-treatment and measurement can be carried out simultaneously during LC/MS measurement, thus enabling more efficient measurement.
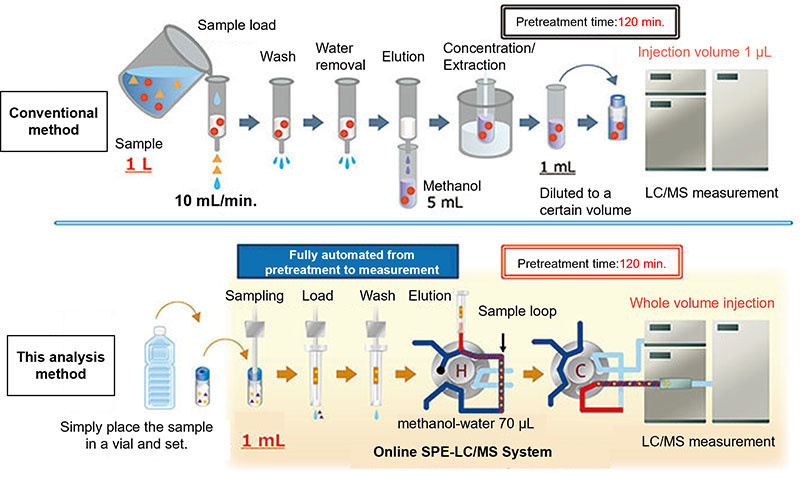
Figure 5. Comparison of this analysis method to the conventional method
3. Analysis method
3-1. Analysis sample
River water
3-2. Standard solution
The following mixture standard solutions manufactured by Fujifilm Wako were used.
3 PFCs Mixture Standard Solution (PFHxS,PFOS,PFOA each 2 µg/mL Methanol Solution), Code No. 162-29071.
3-3. Sample preparation
After shaking, approximately 500 mL of the collected river water was divided into 1 mL portions, placed in a polypropylene vial, and capped with an aluminum foil septum. It was then set into the online SPE-LC/MS system.
3-4. Pretreatment conditions
The pretreatment procedure for the online SPE-LC/MS system SPL-W100 is shown in Figure 6. The SPE column was a dedicated online cartridge Flash-SPE C18 (5 mg) packed with C18, a hydrophobic silica gel.
In the automated pretreatment, 1 mL of sample in a vial was collected and loaded onto the preconditioned solid-phase at a flow rate of 5 µL/sec. to absorb the analyte in the solid-phase. The solid-phase was washed with 250 µL of methanol/water (1/9) and connected to the elution piping. Seventy µL of methanol/water (95/5) was used at a flow rate of 2 µL/sec. to elute the analyte, while 70 µL of water was mixed with the eluate at a flow rate of 2 µL/sec. and collected in the elution loop. The valve was switched for 60 sec. and all the solution in the sample loop containing the analyte was injected into the LC column with the mobile phase.
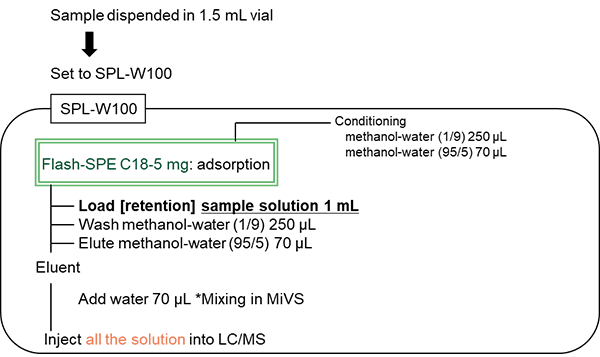
Figure 6. Pretreatment procedure
3-5. Measurement conditions
The LC/MS measurement conditions are shown in Table 1.
For rapid analysis, an Inertsil ODS-3 3 µm, 2.1 mmID x 75 mm (LC column) was used.
Table 1. LC/MS conditions
| [LC conditions] | ||
| Analytical column | : | Inertsil ODS-3, 3 µm, 2.1 mmID x 75 mm |
| Mobile phase | Solvent A : | 2 mM Ammonium acetate solution |
| Solvent B : | 2 mM Ammonium acetate MeOH/acetonitrile (1/1) solution | |
| Flow rate | : | 0.3 mL/min. |
| Gradient | : | B.Conc. 40% (0-1 min.) to 100% (7-9 min.) |
| Column temperature | : | 40°C |
| [MS conditions] | ||
| Ionization | : | ESI Negative |
| MRM | : | PFOA 412.9>169, 412.9>368.9 PFOS 499>79.8, 499>98.9 PFHxS 399>79.8, 399>98.9 |
4. Results
4-1 Calibration curves
Calibration curves obtained by measuring samples prepared by adding mixed standard solutions to ultrapure water at concentrations of 0.5, 1, 2, 5 and 10 parts per trillion (ppt), respectively, by the online SPE-LC/MS system are shown in Figure 7. The correlation coefficients were 0.9998 for PFOA, 0.9998 for PFOS, and 0.9996 for PFHxS, showing good linearity.
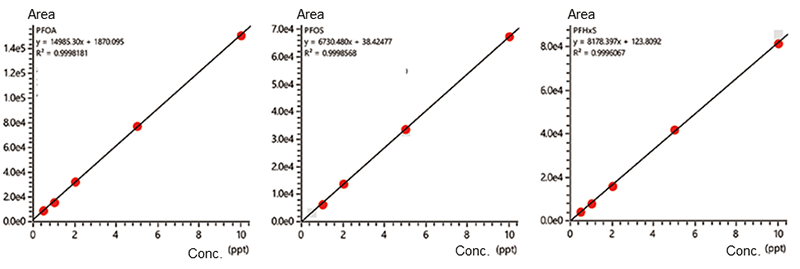
Figure 7. Calibration curve created by online SPE-LC/MS system
4-2. Additive recovery test using river water
Table 2 shows the recovery rates estimated from the peak area values obtained by measuring the collected river water directly with the online SPE-LC/MS system and the peak area values obtained by measuring the river water with a mixture standard solution added to increase the PFAS concentration in the sample by 5 ppt, and the reproducibility of these continuous measurements (n = 6). The respective recovery rates were good: PFOA: 95%, PFOS: 80% and PFHxS: 88%. The respective relative standard deviations (RSDs) were good: PFOA: 2.6%, PFOS: 8.6% and PFHxS: 1.8%. PFOA and PFOS were detected at 5.4 ppt and 2.9 ppt, respectively, in the river water in this study.
Figure 8 shows quantitative ion MRM chromatograms obtained with online SPE-LC/MS system for the standard solution (5 ppt) (A), which is 1/10 of the Japanese provisional indicator value of 50 ppt (0.05 ppm), river water (B), and the blank (C). Good peak shapes were obtained in all cases, indicating that sufficient sensitivity was obtained even at 5 ppt. On the other hand, PFOA was detected at 0.17 ppt in blank (C).
Table 2. Additive recovery test results and reproducibility
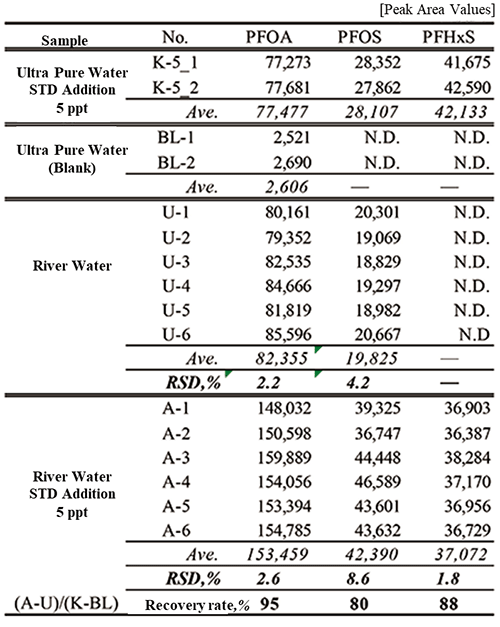
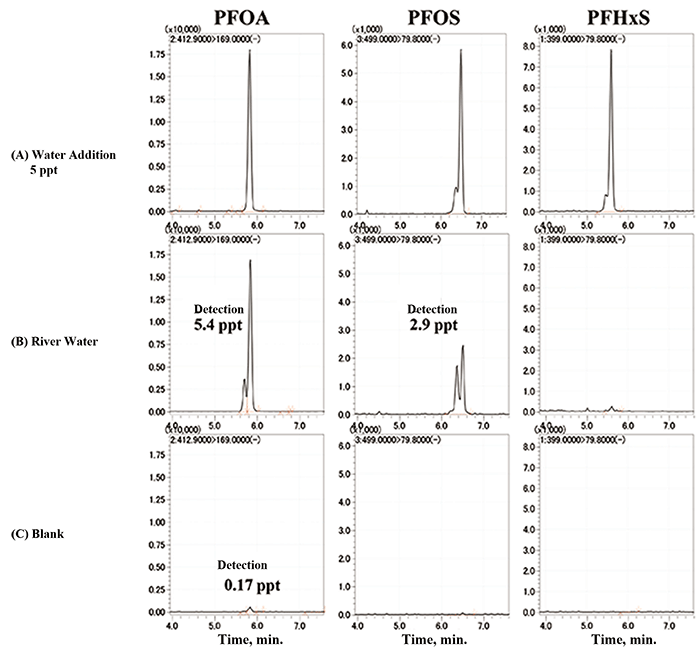
Figure 8. Quantitative ion MRM chromatograms obtained with online SPE-LC/MS system
5. Conclusion
This research has enabled fully automated and rapid analysis of PFAS in river water, from pretreatment to measurement, simply by placing river water in a vial and setting it into an online SPE-LC/MS system.
This is expected not only to reduce the amount of work at the analysis site, but also to improve the accuracy of analysis results by making pretreatment more efficient and less complicated. In addition, the scale-down of pretreatment volumes associated with automation leads to a significant reduction in the amount of organic solvent used, which is several tens of times less than that used in the conventional system. In addition to reducing running costs, this will also reduce the amount of waste solvent used, which will have the effect of reducing environmental impact.
We plan to use this system to establish a simultaneous multi-PFAS analysis method in addition to PFOA, PFOS, and PFHxS in the future.
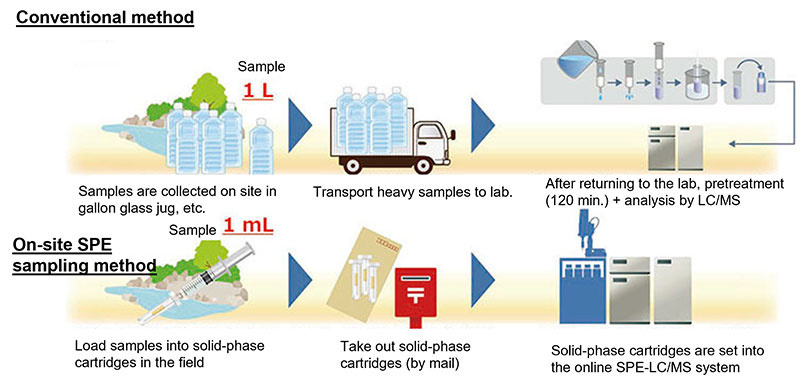
On-site SPE sampling method
In the conventional method, the collected sample (river water) is placed in a large bottle and brought back to the analysis laboratory for pretreatment.
In this method, the sample volume is as small as 1 mL, so the sample can be loaded onto a solid-phase on-site, and the solid-phase is simply brought back to the laboratory and set into the online SPE-LC/MS system. The solid-phase can also be sent by mail, making it possible to analyze river water from distant locations at low cost.
6. References
Sasano, R., Asai, T., Watanabe, J., Ito, R., Akiyama, H.: "Development of an On-line SPE-LC/MS System for PFOA Analysis in River Water", Proceedings of the 29th Symposium of Environmental Chemistry, 658 (2021).




