How to select elemental standard solutions according to the purpose
Elemental impurities are analyzed not only in electronic materials but also in a wide range of areas including pharmaceuticals, foods, and environment (water and soil, etc.). It is very important to ensure the quality of products and to avoid quality problems.
Basic knowledge of elemental analysis you need to know
Trace elemental (metal) analysis uses mainly instrumental analytical procedures. There are various instrumental analytical procedures. The following 3 representative methods are used for trace elemental (metal) analysis.
Atomic absorption spectrometry (AAS)
When the sample solution is heated under high temperature to atomize a measured elemental and when light of wavelength specific to the elemental is passed through it, the atom in the ground state absorbs the light and becomes excited. Quantification is performed based on the absorption (absorbance) of this light.
ICP optical emission spectroscopy (ICP-OES)
A measured elemental (atom) is excited when the sample solution is turned into a spray and introduced into argon plasma excited by high-frequency induction. Elemental specific wavelengths are released as the excited measured elemental returns to its ground state. Quantification is performed based on the intensity of light at this wavelength.
ICP mass spectrometry (ICP-MS)
The sample solution is turned into a spray and introduced into argon plasma excited by high-frequency induction to ionize a measured elemental. Generated ions are detected by a mass spectrometry unit.
Each analytical procedure has the following features.
| AAS | ICP-OES | ICP-MS | |
| Sensitivity | ppb to ppm (frame) ppt to ppb (furnace) |
ppb to % | ppt to ppm |
| Dynamic range | 2 digits | 5 digits | 5 digits |
| Simultaneous multi -elemental analysis |
- | 〇 | 〇 |
| Analysis of rare earth (Zr, Ta, P, B) |
△ | 〇 | 〇 |
| Equipment price | Low price |  |
Expensive |
In recent years, simultaneous multi-elemental analysis has become the mainstream, and the sales performance of metal analyzers shows that especially ICP-MS has been newly introduced.
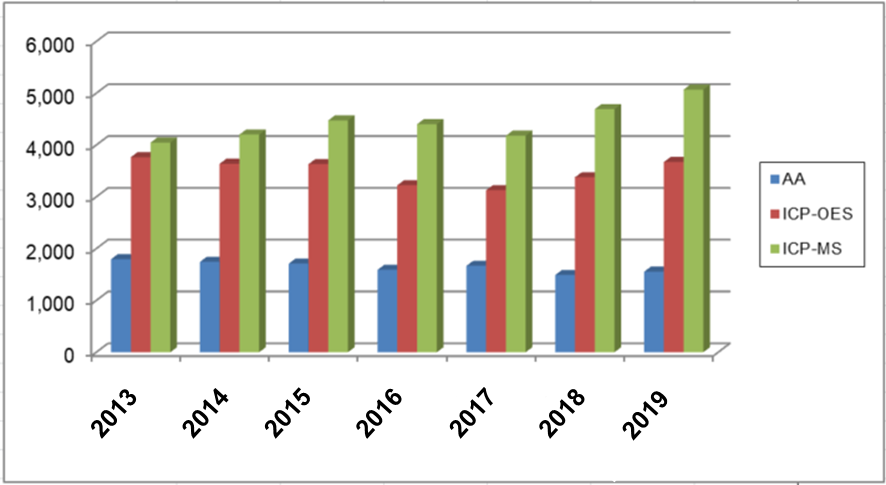
Changes in sales performance of metal analyzers (Source: Scientific Equipment Yearbook 2019)
How to select an elemental standard solution suitable for the purpose
There are various specifications for elemental standard solution (metal standard solution) used for elemental analysis. Isn't it difficult to decide which one should be selected? 3 points for selecting an elemental standard solution are presented below.
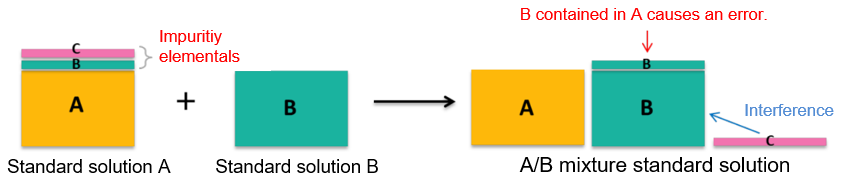
Point 1. Guaranteeing impurity elementals? - What happens if there are impurity elementals? -
As an example, we will consider a case where an elemental standard solution A and elemental standard solution B are mixed to make A/B mixture standard solution.
It is assumed that an elemental (A) to be measured and impurity elementals (B) (C) are contained in the elemental standard solution A. When A/B mixture standard solution is used, the impurity elemental (B) of A elemental standard solution comes out as an "error." In addition, unconcerned "interference" may occur when the A standard solution alone is used. As described above, for simultaneous multi-elemental analysis, in which impurity elementals in the standard solution may affect measurement results, it is recommended to use the "elemental standard solutions for ICP analysis" with the assured concentrations of impurity elementals.
Example 1.
Point 2. Standard solution raw materials are also important factors for selection.
When selecting a standard solution, it is also important to check what raw materials are being used. For AAS, metals (elementals) other than metals (elementals) to be measured may be contained in raw materials.
As an example, the silicon standard solution for AAS contains sodium because it uses Na2SiO3 as a raw material while it uses SiO2 as a raw material for ICP Analysis.
Example 2. Silicon standard solution (Si 1000)
| for Atomic Absorption Spectrometry | for ICP Analysis | |
| Raw material | Na2SiO3 | SiO2 |
Information on raw materials of the standard solutions of FUJIFILM Wako is posted on the website. Click here for more information.
Point 3. Pay attention to combination of mixing
Depending on the type of elemental standard solution, there are combinations that may cause precipitation and volatilization, etc. due to mixing.
Example 3.
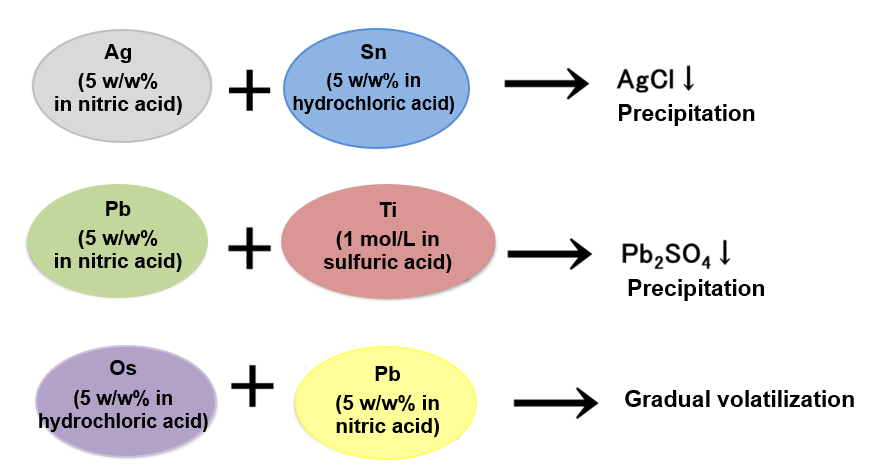
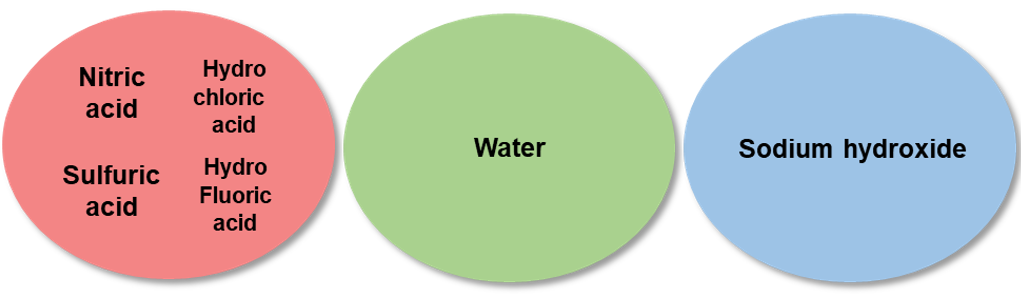
Check the types of acids used in the standard solution to be mixed. Note that elemental standard solutions that may cause precipitation or volatilization, should be prepared separately. The standard solutions differ in pH (acidic, basic). When using mixture standard solutions, it is necessary to check the pH of a single standard solution, to prepare separately standard solutions having different pH, or to use standard solutions having identical pH.
Example 4.
Selection of standard solutions for elemental analysis by specifications
FUJIFILM Wako has 3 specification lineups of elemental standard solutions for each analytical procedure. Please use according to the analytical procedure.
Combination of analytical procedure and recommended standard solution
- ICP-MS, ICP-OES analysis (simultaneous multi-elemental analysis): "for ICP Analysis"
- Atomic absorption spectrometry (AAS): "JCSS", "for Atomic Absorption Spectrometry"
Features of metal standard solutions
| for Atomic Absorption Spectrometry | JCSS | for ICP Analysis | |
| Intended use | Atomic absorption | Atomic absorption | ICP-OES, ICP-MS |
| Concentration measurement method | Various titration methods Weight method |
Various titration methods Ion chromatography |
Various titration methods Ion chromatography ICP-OES |
| Impurity elemental | - | - | Ensuring ppb order using ICP-MS |
| Package insert | - | Calibration certificate | Product instruction (with impurity elemental information) |
| Features | - |
|
|
Summary
- Representative trace elemental (metal) analytical procedures include atomic absorption spectrometry (AAS), ICP optical emission spectroscopy (ICP-OES), and ICP mass spectrometry (ICP-MS).
- Standard solutions for elemental analysis with specifications appropriate for analytical procedures are available, and there are 3 points for selection.
- Ensure the assurance of impurity elementals.
- Check raw materials.
- When using after mixing, check the compatibility and pH of the standard solution.
FUJIFILM Wako has 3 specification lineups of "metal standard solutions (elemental standard solutions)" for each analytical procedure. In addition, "high-purity acids" with less metal impurities are also available. We provide total support for trace elemental analysis.




